CAS Number 14987-04-3 Anhydrous and 39365-87-2 for Hydrate, Magnesium Trisilicate Anhydrous Hydrate USP BP Ph Eur FCC Food Grade Manufacturers Exporters







CAS Number 14987-04-3 Anhydrous and 39365-87-2 for Hydrate, Magnesium Trisilicate Anhydrous Hydrate Manufacturer Exporter
For Properties Specifications of Magnesium Trisilicate Anhydrous Hydrate Click Properties, Specifications of Magnesium Trisilicate Anhydrous Hydrate Manufacturer.
For Uses of Magnesium Trisilicate Anhydrous Hydrate Click Uses of Magnesium Trisilicate Anhydrous Hydrate Manufacturer.
For For SDS MSDS Sheet of Magnesium Trisilicate Anhydrous Hydrate Click SDS Safety Data Sheet MSDS Sheet of Magnesium Trisilicate Anhydrous Hydrate Manufacturer.
The Properties and Specifications of Magnesium Trisilicate Anhydrous Hydrate:
Magnesium Trisilicate USP Grade specifications
2MgO3SiO2xH2O -- (anhydrous) 260.86
Silicic acid (H4Si3O8), magnesium salt (1:2), hydrate.
Magnesium silicate hydrate (Mg2Si3O8xH2O) CAS 39365-87-2
Anhydrous CAS 14987-04-3
Magnesium Trisilicate is a compound of Magnesium Oxide and silicon dioxide with varying proportions of water. It contains not less than 20.0 percent of magnesium oxide (MgO) and not less than 45.0 percent of silicon dioxide (SiO2).
Identification:
A: Mix about 500 mg with 10 mL of 3 N hydrochloric acid, filter, and neutralize the filtrate to litmus paper with 6 N ammonium hydroxide: the neutralized filtrate responds to the tests for Magnesium.
B: Prepare a bead by fusing a few crystals of sodium ammonium phosphate on a platinum loop in the flame of a Bunsen burner. Place the hot, transparent bead in contact with Magnesium Trisilicate, and again fuse: silica floats about in the bead, producing, upon cooling, an opaque bead with a web-like structure.
Water: Weigh accurately about 1 g in a tared platinum crucible provided with a cover. Gradually apply heat to the crucible at first, then strongly ignite to constant weight: it loses between 17.0% and 34.0% of its weight.
Soluble salts: Boil 10.0 g with 150 mL of water for 15 minutes. Cool to room temperature, allow the mixture to stand for 15 minutes, filter with the aid of suction, transfer the filtrate to a 200-mL volumetric flask, dilute with water to volume, and mix. Evaporate 50.0 mL of this solution, representing 2.5 g of the Trisilicate, in a tared platinum dish to dryness, and ignite gently to constant weight: the weight of the residue does not exceed 38.0 mg (1.5%).
Chloride: A 20-mL portion of the diluted filtrate prepared in the test for Soluble salts, representing 1 g of Magnesium Trisilicate, shows no more chloride than corresponds to 0.75 mL of 0.020 N hydrochloric acid (0.055%).
Sulfate: Treat the residue obtained in the test for Soluble salts with 2 mL of hydrofluoric acid, and evaporate on a steam bath to dryness. Mix the residue with water, transfer to a filter, and wash, using approximately 50 mL of water for the complete procedure. Heat the filtrate to boiling, and add 0.1 mL of hydrochloric acid and 5 mL of barium chloride TS. Maintain the mixture near its boiling point for 1 hour, filter, wash the precipitate thoroughly with water, dry, and ignite to constant weight: the weight of the residue does not exceed 30 mg (0.5%).
Free alkali: Add 2 drops of PhPh TS to 20 mL of the diluted filtrate prepared in the test for Soluble salts, representing 1 g of the Trisilicate: if a pink color is produced, not more than 1.0 mL of 0.10 N hydrochloric acid is required to discharge it.
Arsenic: 8 ppm.
Heavy metals: the limit is 0.003%.
Acid-consuming capacity: Weigh accurately about 200 mg into a glass-stoppered, 125-mL conical flask. Add 30.0 mL of 0.1 N hydrochloric acid VS and 20.0 mL of water. Place the flask in a bath maintained at 37 , and shake the mixture occasionally during a period of 4 hours but leave the mixture undisturbed during the last 15 minutes of the heating period. Cool to room temperature. To 25.0 mL of the supernatant add methyl red TS, and titrate the excess acid with 0.1 N sodium hydroxide VS. One g of Magnesium Trisilicate, calculated on the anhydrous basis, consumes not less than 140 mL and not more than 160 mL of 0.10 N hydrochloric acid.
Ratio of SiO2 to MgO: between 2.10 and 2.37.
Magnesium Trisilicate BP Ph Eur Grade Specifications
DEFINITION
It has a variable composition corresponding approximately to Mg2Si3O8,xH2O.
Content:
magnesium oxide: minimum 29.0 per cent (ignited substance),
silicon dioxide: minimum 65.0 per cent (ignited substance).
CHARACTERS
Appearance: White or almost white powder.
Solubility: Practically insoluble in water and in ethanol (96 per cent).
IDENTIFICATION
A. 10.25 g gives the reaction of silicates.
B. 1 ml of solution S (see Tests) neutralized with dilute sodium hydroxide solution gives the reaction of magnesium.
TESTS
Solution S: To 2.0 g add a mixture of 4 ml of nitric acid and 4 ml of distilled water. Heat to boiling with frequent shaking. Add 12 ml of distilled water and allow to cool. Filter or centrifuge to obtain a clear solution and dilute to 20 ml with distilled water.
Alkalinity: To 10.0 g in a 200 ml conical flask, add 100.0 g of water and heat on a water-bath for 30 min. Allow to cool and make up to the initial mass with water R. Allow to stand and filter or centrifuge until a clear liquid is obtained. To 10 ml of this liquid add 0.1 ml of PhPh solution. Not more than 1.0 ml of 0.1 M hydrochloric acid is required to change the colour of the indicator.
Water-soluble salts: Maximum 1.5 per cent. In a platinum dish, evaporate to dryness on a water-bath 20.0 ml of the liquid obtained in the test for alkalinity. The residue, ignited to constant mass at 900±50C, weighs a maximum of 30 mg.
Chlorides: Maximum 500 ppm.
Sulphates: Maximum 0.5 per cent.
Arsenic: Maximum 4 ppm, determined on 2.5 ml of solution S.
Heavy metals: Maximum 40 ppm.
Loss on ignition: 17 per cent to 34 per cent, determined on 0.5 g by ignition to constant mass at 900± 50C in a platinum crucible.
Acid-absorbing capacity: Suspend 0.25 g in 0.1 M hydrochloric acid , dilute to 100.0 ml with the same acid and allow to stand for 2 h in a water-bath at 37 ± 0.5 °C, with frequent shaking. Allow to cool. To 20.0 ml of the supernatant solution add 0.1 ml of bromophenol blue solution and titrate with 0.1 M sodium hydroxide until a blue colour is obtained. The acid-absorbing capacity is not less than 100.0 ml of 0.1 M hydrochloric acid per gram.
Magnesium Silicate FCC Food Grade specifications
Synthetic Magnesium Silicate
INS: 553(i) CAS 1343-88-0
DESCRIPTION
Magnesium Silicate occurs as a very fine, white powder free from grittiness. It is a synthetic, usually amorphous form of magnesium silicate in which the molar ratio of magnesium oxide (MgO) to silicon dioxide (SiO2) is approximately 2:5. It is insoluble in water and in alcohol, but is readily decomposed by mineral acids. The pH of a 1:10 slurry is between 7.0 and 10.8.
Function: Anti-caking agent; filter aid.
REQUIREMENTS
Identification:
A. Mix about 500 mg of sample with 10 mL of 2.7 N hydrochloric acid, filter, and neutralize the filtrate to litmus paper with 6 N ammonium hydroxide. The neutralized filtrate gives positive tests for Magnesium.
B. Prepare a bead by fusing a few crystals of sodium ammonium phosphate on a platinum loop in the flame of a Bunsen burner. Place the hot, transparent bead in contact with a sample, and again fuse. Silica floats about in the bead, producing, upon cooling, an opaque bead with a web-like structure.
Assay: Not less than 15.0% of MgO and not less than 67.0% of SiO2, calculated on the ignited basis.
Fluoride: Not more than 10 mg/kg.
Free Alkali (as NaOH): Not more than 1%.
Lead: Not more than 5 mg/kg.
Loss on Drying and Loss on Ignition: Not more than the percentage stated or within the range claimed by the vendor.
Soluble Salts: Not more than 3.0%.
The Uses of Magnesium Trisilicate Anhydrous Hydrate:
Magnesium Trisilicate treats occasional heartburn, indigestion, upset stomach, or other conditions caused by too much stomach acid. It works by reducing the amount of acid in the stomach. Magnesium trisilicate is an antacid used for the symptomatic treatment of peptic ulcers. Magnesium trisilicate mixture is an antacid with slow neutralising action and mild laxative action. Magnesium trisilicate is an inorganic compound that is used as a food additive. The additive is frequently used by fast food chains to absorb fatty acids.
The MSDS-SDS Hazard Statement of Magnesium Trisilicate Anhydrous Hydrate:
Magnesium Trisilicate SDS, Safety Data Sheet
MSDS Sheet, Material Safety Data Sheet 14-Jan-23
Section 1: Chemical Product and Company Identification
Product Name & Other Names: Magnesium Trisilicate or di-Magnesium trisilicate or Magnesii trisilicas.
CAS Number: 14987-04-3 for anhydrous or 39365-87-2 for hydrate
EINECS EC Code: 239-076-7
Chemical Formula: Mg2O8Si3 as anhydrous
Molecular Weight: 260.86 as anhydrous
Relevant uses and uses advised against (if any) : Industrial Manufacturing.
Section 2: Hazards Identification
GHS, Globally Harmonized System Classification in accordance with 29 CFR 1910
Classification according to Regulation (EC) No 1272/2008
Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008.
This substance is not classified as dangerous according to Directive 67/548/EEC.
Labeling according to GHS & Regulation (EC) No 1272/2008
GHS Label Elements NONE |
Signal Word: None
Precautionary statements:
P261: Avoid breathing dust/fume/gas/mist/vapors/spray.
P262: Do not get in eyes, on skin, or on clothing.
P281: Use personal protective equipment as required.
P302+P352: IF ON SKIN: Wash with plenty of soap and water.
P304+P340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.
P337+313: If eye irritation persists get medical advice/attention.
Section 3: Composition and Information on Ingredients
Product Name & Other Names: Magnesium Trisilicate or di-Magnesium trisilicate or Magnesii trisilicas.
CAS Number: 14987-04-3 for anhydrous or 39365-87-2 for hydrate
EINECS EC Code: 239-076-7
Section 4: First Aid Measures
Always seek medical advice after the first aid treatment.
Skin: Rinse with water. Soap may be used. Seek Medical Aid.
Eyes: Wash eyes with plenty of water for at least 15 minutes, lifting lids occasionally. Seek Medical Aid.
Inhalation: Remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Ingestion: If swallowed, induce vomiting immediately after giving two glasses of water. Never give anything by mouth to an unconscious person.
Notes to Physician: Treat symptomatically.
Section 5: Fire and Explosion Data
Flammability of the Product: Non-flammable.
Products of Combustion: Magnesium oxide, Silicon Oxides.
Fire Fighting Media and Instructions: Use extinguishing measures that are appropriate to local circumstances and the surrounding environment.
Special Information: In the event of a fire, wear full protective clothing and NIOSH-approved self-contained breathing apparatus with full face piece operated in the pressure demand or other positive pressure mode. At high temperatures under fire conditions, it may produce toxic or irritating fumes. Fire-extinguishing work is done from the windward and the suitable fire-extinguishing method according to the surrounding situation is used. Uninvolved persons should evacuate to a safe place.
Section 6: Accidental Release Measures
Personal precautions, protective equipment, and emergency procedures: Ventilate area of leak or spill. Avoid breathing dust/fumes/gas/mist/vapors/spray. Use individual protective equipment (waterproof boots, suitable protective clothing, safety glasses, etc.). Restrict unprotected personnel from the area. Prevent any contact with hot surfaces. Do not approach facing the wind. Do not touch the spilled material.
Environmental precautions: Do not let the product enter drains, soil, or water sources.
Methods and materials used for containment Cleanup procedures and Storage: Contain spilled material. Cover with an inert, non-combustible absorbent material, (e.g. sand, earth, diatomaceous earth, vermiculite). Vacuum or sweep-up and remove to an approved disposal container. Do not inhale vapors, mist, or gas. Avoid dust formation. Finish cleaning by spreading water on the contaminated surface and allow to evacuate as per law.
Section 7: Handling and Storage
Precautions for safe handling: Apply according to good manufacturing and industrial hygiene practices. Ensure proper ventilation. Wash thoroughly after handling. Do not drink, eat, or smoke while handling. Avoid contact with skin, eyes, and clothing. Minimize dust generation. Avoid breathing dust/fumes/gas/mist/vapors/spray. Keep container tightly closed. Avoid ingestion and inhalation. Use individual protective equipment (waterproof boots, suitable protective clothing, safety glasses, etc.).
Conditions for safe storage, including any incompatibilities: Store in cool, dry, and ventilated area away from heat sources and protected from sunlight in tightly closed original container. Keep air contact to a minimum. Store protected from heat, sparks and ignition sources and incompatible materials. Avoid contact with skin and eyes. Avoid inhalation of dust/mist/vapor. Do not store with incompatible materials like strong oxidizing agents and acids.
Section 8: Exposure Controls/Personal Protection
Engineering Controls: Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended exposure limits.
Ventilation System: A system of local and/or general exhaust is recommended to keep employee exposures as low as possible.
Personal Respirators (NIOSH Approved): For conditions of use where exposure to dust or mist is apparent and engineering controls are not feasible, a particulate respirator may be worn.
Skin Protection: Wear protective gloves and clean body-covering clothing.
Eye Protection: Use chemical safety goggles and/or full face shield where dusting or splashing of solutions is possible. Maintain eye wash fountain and quick-drench facilities in work area.
Other Control Measures: Maintain good housekeeping in work area. Handle in accordance with good industrial hygiene and safety practice.
Section 9: Physical and Chemical Properties
Physical state and appearance: Solid white to off-white powder or granules.
Odor: Odorless.
Odor threshold: Not available.
pH: Not available.
Relative density: around 2.5
Melting point/freezing point: Not available.
Initial boiling point and boiling range: Not available.
Flash point: Not available.
Auto-ignition temperature: Not available.
Decomposition temperature: Not available.
Upper/lower flammability or explosive limits: Not available.
Vapor pressure: Not available.
Vapor density: Not available.
Evaporation rate: Not available.
Flammability (solid, gas): Not available.
Partition coefficient: n-octanol/water: Not available.
Solubility(ies): Not available.
Viscosity: Not available.
Section 10: Stability and Reactivity Data
Stability: It is stable in room temperature in closed containers under normal storage & handling.
Conditions of instability: Incompatible materials
Incompatibility with various substances: Strong oxidizing agents, acids.
Polymerization: Will not occur.
Section 11: Toxicological Information
Toxicity to Animals: No data available.
Carcinogenic Effects: Not a reported carcinogen by IARC, NTP, ACGIH, OSHA.
Mutagenic Effects: Not available.
Developmental Toxicity: Not available.
Reproductive Effects: No information available.
Section 12: Ecological Information
Ecotoxicity: No specific data or information available regarding environmental impact. High alkalinity of material when in solution or suspension may pose localized adverse effects.
Persistence and Degradability: No information available.
Mobility: No information available.
Bioaccumulation/ Accumulation: No information available.
Results of PBT and vPvB assessment: No data available for assessment.
Section 13: Disposal Considerations
Waste Disposal: Waste must be disposed of in accordance with federal, state, and local environmental control regulations.
Section 14: Transport Information
DOT USA, TDG Canada & ADR/RID Europe: Not controlled.
IMDG/IMO: Not controlled.
IATA: Not controlled.
Section 15: Other Regulatory Information
USA Federal and State Regulations:
California Prop. 65 Components: Not listed.
DISCLAIMER: The information and recommendations set forth herein are presented in good faith and believed correct as of the date hereof. It is compiled from various sources and it is not necessarily all inclusive nor fully adequate in every circumstance. In addition, these suggestions should not be confused with nor followed in violation of applicable laws, regulations, rules or insurance requirements applicable. This MSDS sheet is intended only as a guide to the appropriate precautionary handling of the material by a properly trained person using this product. Individuals receiving the information must exercise their independent judgment in determining its appropriateness for a particular purpose.
Anmol Chemicals & Pharmaceuticals Pvt. Ltd. is an off-shoot of Anmol Chemicals Taloja. It is located in MIDC Taloja and it is manufacturing pharmaceutical grades of API, Excepients, Food grade and Reagent grade chemicals. Anmol Chemicals & Pharmaceuticals Pvt. Ltd. is a several decades old group of companies, engaged in manufacturing, supplying, distributing, wholesale supplies for actual users, retail or small pack supplies for research and development chemicals, fine and speciality chemicals, pharmaceutical excipients, mineral fortifiers in chemically pure, Analytical reagent grade, IP BP USP Ph Eur EP JP and other pharmaceutical grade monograph including FCC Food grade chemicals and Nutraceuticals, Mineral Fortifiers at best prices.
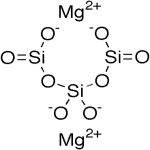
Magnesium Trisilicate Anhydrous Hydrate Structure
CAS Number 14987-04-3 Anhydrous and 39365-87-2 for Hydrate, Magnesium Trisilicate Anhydrous Hydrate Manufacturer Exporter
ANMOL CHEMICALS & PHARMACEUTICALS Pvt. Ltd.
India, USA, Europe, UAE
TELEPHONE: +912223770100
Navi Mumbai, INDIA
e-mail: info(At the rate i.e. @)anmol.org
Copyright. 11-dec-24
We manufacture:

