CAS Number 129722-12-9, Aripiprazole BP Ph Eur EP IP USP Grade Manufacturers Exporters







CAS Number 129722-12-9, Aripiprazole BP Ph Eur EP IP USP Manufacturer Exporter
For Properties Specifications of Aripiprazole Click Properties, Specifications of Aripiprazole Manufacturer.
For Uses of Aripiprazole Click Uses of Aripiprazole Manufacturer.
For For SDS MSDS Sheet of Aripiprazole Click SDS Safety Data Sheet MSDS Sheet of Aripiprazole Manufacturer.
The Properties and Specifications of Aripiprazole:
Aripiprazole BP Ph Eur Grade:
C23H27Cl2N3O2 --- 448.4 CAS 129722-12-9
Action and use: Dopamine D2 receptor antagonist; neuroleptic.
DEFINITION
7-[4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butoxy]-3,4-dihydroquinolin-2(1H)-one.
Content: 98.0 per cent to 102.0 per cent (dried substance).
CHARACTERS
Appearance: White or almost white crystals or crystalline powder.
Solubility: Practically insoluble in water, soluble in methylene chloride, very slightly soluble in ethanol (96 per cent).
It shows polymorphism.
IDENTIFICATION
Infrared absorption spectrophotometry
Related substances: To pass the test.
Loss on drying: Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105C for 3 h.
Sulfated ash: Maximum 0.1 per cent, determined on 1.0 g.
Bacterial endotoxins: Less than 5 IU/mg, if intended for use in the manufacture of parenteral preparations without a further appropriate procedure for the removal of bacterial endotoxins.
Dissolve 1.0 mg of the substance to be examined in 20 mL of a 5.17 g/L solution of hydrochloric acid.
Storage: Protected from light. If the substance is sterile, store it in a sterile, airtight, tamper-proof container.
Aripiprazole USP Grade Specifications:
C23H27Cl2N3O2 448.39
2(1H)-Quinolinone, 7-[4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy]-3,4-dihydro-;
7-[4-[4-(2,3-Dichlorophenyl)-1-piperazinyl]butoxy]-3,4-dihydrocarbostyril CAS 129722-12-9].
UNII: 82VFR53I78
DEFINITION
Aripiprazole contains NLT 98.0% and NMT 102.0% of aripiprazole (C23H27Cl2N3O2), calculated on the dried basis.
IDENTIFICATION
A. Infrared Absorption
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
Residue on Ignition: NMT 0.1%
Organic Impurities: To pass the test.
Aripiprazole related compound G: 0.1% maximum.
Aripiprazole related compound F: 0.1% maximum.
Aripiprazole 4,4’ dimer: 0.1% maximum.
Any other individual impurity: 0.1% maximum.
Total impurities: 0.5% maximum.
Loss on Drying: Dry at 105C for 3 h. Aceptance criteria: NMT 0.5%
Packaging and Storage: Preserve in tight containers. Store at controlled room temperature.
Aripiprazole is also manufacture in EP IP Grade.
The Uses of Aripiprazole:
Aripiprazole is an atypical antipsychotic drug.
The MSDS-SDS Hazard Statement of Aripiprazole:
Aripiprazole SDS, Safety Data Sheet
MSDS Sheet, Material Safety Data Sheet 10-Nov-24
1. Product Identification
Product Name & Other Names: Aripiprazole.
CAS No.: 129722-12-9
EINECS EC Number: 603-355-5
Relevant uses and uses advised against (if any): Industrial Manufacturing.
Suppliers: As per letterhead.
2. Hazards Identification
GHS, Globally Harmonized System Classification in accordance with 29 CFR 1910
Classification according to Regulation (EC) No 1272/2008
Acute toxicity, oral Category 4, H302
Labeling according to Regulation (EC) No 1272/2008
GHS Label Elements  Harmful |
Signal Words: Warning
Hazard statements:
H302: Harmful if swallowed.
Precautionary statements:
P262: Do not get in eyes, on skin, or on clothing.
P280: Wear protective gloves/protective clothing/eye protection/face protection.
P270: Do not eat, drink or smoke when using this product.
P330: Rinse mouth.
P362: Take off contaminated clothing and wash before reuse.
P301+312: IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell.
P302+P352: IF ON SKIN: Wash with plenty of soap and water.
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.
P332+313: If skin irritation occurs: Get medical advice/attention.
P337+P313 If eye irritation persists: Get medical advice/ attention.
3. Composition/Information on Ingredients
Product Name & Other Names: Aripiprazole.
CAS No.: 129722-12-9
EINECS EC Number: 603-355-5
4. First Aid Measures
Inhalation: Remove to fresh air. Give oxygen if breathing is difficult; give artificial respiration if breathing has stopped. Keep person warm and quiet; get medical attention.
Ingestion: Induce vomiting immediately as directed by medical personnel. Never give anything by mouth to an unconscious person.
Skin Contact: Immediately flush skin with plenty of soap and water for at least 15 minutes. Remove contaminated clothing and shoes. Wash clothing before reuse. Thoroughly clean shoes before reuse. Get medical attention if irritation develops.
Eye Contact: Immediately flush eyes with plenty of water for at least 15 minutes, lifting upper and lower eyelids occasionally. Get medical attention.
5. Fire Fighting Measures
Fire: May become a fire hazard at high temperatures.
Fire Extinguishing Media: Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture: Carbon oxides, Nitrogen oxides (NOx), Hydrogen chloride gas, Chlorine compounds.
Special Information In the event of a fire, wear full protective clothing and NIOSH-approved self-contained breathing apparatus with full face piece operated in the pressure demand or other positive pressure mode.
6. Accidental Release Measures
Personal precautions, protective equipment, and emergency procedures: Use personal protective equipment. Avoid dust formation. Avoid breathing dust/fumes/gas/mist/vapors/spray. Use individual protective equipment (waterproof boots, suitable protective clothing, safety glasses, etc.).
Environmental precautions: Do not let the product enter drains, soil, or water sources.
Methods and materials used for containment cleanup procedures and Storage: Contain spilled material. Cover with an inert, non-combustible absorbent material, (e.g., sand, earth, diatomaceous earth, vermiculite). Vacuum or sweep-up and remove to an approved disposal container.
7. Handling and Storage
Precautions for safe handling: Apply according to good manufacturing and industrial hygiene practices. Ensure proper ventilation. Wash thoroughly after handling. Do not drink, eat, or smoke while handling. Avoid contact with skin, eyes, and clothing. Minimize dust generation. Avoid breathing dust/fumes/gas/mist/vapors/spray. Keep container tightly closed. Avoid ingestion and inhalation. Use individual protective equipment (waterproof boots, suitable protective clothing, safety glasses, etc.).
Conditions for safe storage, including any incompatibilities: Store in cool, dry, and ventilated area away from heat sources and protected from sunlight in tightly closed original container. Keep air contact to a minimum. Do not leave the material container open. Store protected from heat, sparks and ignition sources and incompatible materials. Avoid inhalation of dust/mist/vapor. Do not store with incompatible materials like strong oxidizing agents. Avoid dust formation and control ignition sources. Storage: Preferably 2C to 8C. OK to freeze. Protected from light.
8. Exposure Controls/Personal Protection
Airborne Exposure Limits: Contains no substances with occupational exposure limit values.
Ventilation System: A system of local and/or general exhaust is recommended to keep employee exposures as low as possible.
Personal Respirators (NIOSH Approved): For conditions of use where exposure to dust or mist is apparent and engineering controls are not feasible, a particulate respirator may be worn.
Skin Protection: Wear protective gloves and clean body-covering clothing.
Eye Protection: Use chemical safety goggles and/or full-face shield where dusting or splashing of solutions is possible. Maintain eye wash fountain and quick-drench facilities in work area.
Other Control Measures: Maintain good housekeeping in work area. Handle in accordance with good industrial hygiene and safety practice. Wash hands after handling.
9. Physical and Chemical Properties
Appearance: White to off-white, crystalline powder.
Odor: No data found.
Odor threshold: No data found.
pH: No data found.
Relative density: about 1.3
Boiling Point: 646C.
Melting Point: 137C to 140C literature.
Flash point: No data found.
Auto-ignition temperature: No data found.
Decomposition temperature: No data found.
Upper/lower flammability or explosive limits: No data found.
Vapor pressure: No data found.
Vapor density: No data found.
Evaporation rate: No data found.
Flammability (solid, gas): No data found.
Partition coefficient: n-octanol/water: No data found.
Solubility: Freely soluble in dichloromethane. Insoluble in water.
Viscosity: No data found.
10. Stability and Reactivity
Stability: Stable under recommended conditions of use and storage.
Hazardous Decomposition Products: Nitrogen & carbon oxides along with chlorine compounds.
Hazardous Polymerization: Will not occur.
Incompatibilities: Strong oxidizing agents.
Conditions to Avoid: Heat, light, flame, ignition sources, dusting, and incompatibles.
11. Toxicological Information
LD50 Oral - Rat - 953 mg/kg.
Carcinogenic Effects: Not a reported carcinogen by IARC, NTP, ACGIH, OSHA.
Mutagenic Effects: No data found.
Developmental Toxicity: No data found.
Reproductive Effects: No data found.
12. Ecological Information
Toxicity to fish: LC50 - Fish - > 120 mg/l - 96 h.
Persistence and Degradability: No data found.
Bioaccumulation/ Accumulation: No data found.
Results of PBT and vPvB assessment: No data available for assessment.
13. Disposal Considerations
Whatever cannot be saved for recovery or recycling should be managed in an appropriate and approved waste disposal facility. Follow all the pollution control norms.
14. Transport Information
DOT USA, TDG and ADR/RID Europe: Not controlled.
IATA and IMDG/IMO: Not controlled.
15. Regulatory Information
USA:
SARA 311/312 Hazardous Categorization: See section 2.
California Proposition 65: Not listed.
16. Other Information
Disclaimer:
**************************
Our company provides this MSDS sheet in good faith but makes no representation as to its comprehensiveness or accuracy. This SDS sheet is intended only as a guide to the appropriate precautionary handling of the material by a properly trained person using this product. The above information has been compiled from various sources and has the possibility of discrepancy and being out-dated information. Individuals receiving the information must exercise their independent judgment and do further search in determining its appropriateness for a particular purpose. In no case shall our company be liable to loss or damages by the product user.
**************************
Anmol Chemicals & Pharmaceuticals Pvt. Ltd. is an off-shoot of Anmol Chemicals Taloja. It is located in MIDC Taloja and it is manufacturing pharmaceutical grades of API, Excepients, Food grade and Reagent grade chemicals. Anmol Chemicals & Pharmaceuticals Pvt. Ltd. is a several decades old group of companies, engaged in manufacturing, supplying, distributing, wholesale supplies for actual users, retail or small pack supplies for research and development chemicals, fine and speciality chemicals, pharmaceutical excipients, mineral fortifiers in chemically pure, Analytical reagent grade, IP BP USP Ph Eur EP JP and other pharmaceutical grade monograph including FCC Food grade chemicals and Nutraceuticals, Mineral Fortifiers at best prices.
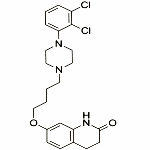
Aripiprazole Structure
CAS Number 129722-12-9, Aripiprazole Manufacturer Exporter
ANMOL CHEMICALS & PHARMACEUTICALS Pvt. Ltd.
India, USA, Europe, UAE
TELEPHONE: +912223770100
Navi Mumbai, INDIA
e-mail: info(At the rate i.e. @)anmol.org
Copyright. 11-dec-24
We manufacture:

