CAS Number 8047-67-4, Iron Sucrose USP BP Ph Eur Grade Manufacturers Exporters







CAS Number 8047-67-4, Iron Sucrose Manufacturer Exporter
For Properties Specifications of Iron Sucrose Click Properties, Specifications of Iron Sucrose Manufacturer.
For Uses of aaa Click Uses of Iron Sucrose Manufacturer.
For For SDS MSDS Sheet of aaa Click SDS Safety Data Sheet MSDS Sheet of Iron Sucrose Manufacturer.
The Properties and Specifications of Iron Sucrose:
Iron Sucrose Injection USP Grade:
Iron Sucrose Injection is a sterile, colloidal solution of ferric hydroxide in complex with Sucrose in Water for Injection. It contains not less than 95.0 percent and not more than 105.0 percent of the labeled amount of iron. Sodium Hydroxide may be added to adjust the pH. It contains no antimicrobial agent, chelating agent, dextran, gluconate, or other added substances.
Packaging and storage: Preserve in single-dose containers of Type I glass. Store at controlled room temperature. Do not freeze.
Identification:
A: Iron: To 2.5 mL of Injection add 17.5 mL of water and 5 mL of hydrochloric acid, mix, and heat for 5 minutes in a boiling water bath. Cool, add drop-wise 13.5 N ammonium hydroxide until no further precipitation of ferric hydroxide occurs, and filter. Wash the precipitate with water to remove excess ammonium hydroxide, dissolve the precipitate in a minimum volume of 2 N hydrochloric acid, and add sufficient water to make a volume of 20 mL. To 3 mL of the solution so obtained add 1 mL of 2 N hydrochloric acid and 1 mL of potassium thiocyanate: the resulting solution (Solution 1) is red. To 1 mL of Solution 1 add 5 mL of amyl alcohol or ethyl ether, shake, and allow to stand: the organic layer is pink. To a separate 1-mL aliquot of Solution 1 add 2 mL of mercuric chloride: the red color is discharged [iron (III) salts].
B: Sucrose: The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay for sucrose.
C: Molecular weight determination: To pass the test
The molecular weight distribution curve obtained for the Injection conforms to the following parameters:
MW = 34,000–60,000 Da,
MN = not less than 24,000 Da, and
MW / MN = not more than 1.7.
Specific gravity: not less than 1.135 and not more than 1.165 at 20C.
Bacterial endotoxins: not more than 3.7 USP Endotoxin Units per mg of iron contained in the Injection.
Alkalinity: Transfer 5 mL of Injection to a suitable vessel, and titrate with 0.1 N hydrochloric acid with constant stirring to a pH of 7.4. Record the volume of 0.1 N hydrochloric acid consumed, and calculate the alkalinity of the Injection as the volume of acid, in mL, consumed per mL of Injection. Not less than 0.5 mL and not more than 0.8 mL of 0.1 N hydrochloric acid is consumed per mL of Injection.
pH: between 10.5 and 11.1 at 20 .
Osmolarity: not less than 1150 mOsmol per L and not more than 1350 mOsmol per L for the Injection. The solution for test is prepared by diluting the Injection 1 in 10.
Absence of low-molecular weight Fe(II) and Fe(III) complexes: To pass the test.
Particulate matter: To meet the requirement.
Limit of iron (II): Not more than 0.4% (w/v) of iron (II) is found.
Content of chloride: The chloride content of the Injection is not less than 0.012% and not more than 0.025%.
Assay for sucrose: To meet the requirement.
Assay for iron: To meet the requirement.
Iron Sucrose Injection BP Ph Eur Grade:
Action and use: Treatment of iron-deficiency anaemia.
DEFINITION
Iron Sucrose Injection is a sterile colloidal solution containing a complex of iron(III) hydroxide with sucrose of average molecular weight between 34000 and 60000.
PRODUCTION
Iron Sucrose Injection is produced by a method of manufacture designed to provide an iron-sucrose complex with appropriate iron absorption characteristics. This may be confirmed for routine control purposes by the use of an appropriate combination of physico-chemical tests, subject to the agreement of the competent authority.
A suitable test is carried out to demonstrate (1) the amount of iron(II) present in the injection is not more than 0.4% w/v of the total iron content and (2) there are no low molecular weight complexes in the injection.
The injection complies with the requirements stated under Parenteral Preparations and with the following requirements.
Content of iron, Fe: 95.0 to 105.0% of the stated amount.
Content of sucrose: 90.0 to 110.0% of the stated amount.
IDENTIFICATION
A. To a quantity of the injection containing the equivalent of 20 mg of iron, add 20 mL of water and 5 mL of hydrochloric acid and boil for 5 minutes. Cool, add an excess of 13.5M ammonia and filter. Wash the precipitate with water, dissolve in the minimum volume of 2M hydrochloric acid and add sufficient water to produce 20 mL The resulting solution yields reaction B characteristic of iron salts.
B In the Assay for Sucrose, the retention time of the principal peak in the chromatogram obtained with solution (1) corresponds to that of the principal peak in the chromatogram obtained with solution (2).
C. Complies with the test for Molecular weight determination.
TESTS
Alkalinity: pH, 10.5 to 11.0.
Osmolality: The osmolality, of the injection is 1150 mosmol/kg to 1350 mosmol/kg.
Clarity of solution: To a quantity of the injection containing 10 mg of iron, add 100 mL of water and adjust to pH 6.0 with 0.1M hydrochloric acid. The solution must not have any turbidity. Add 0.1M hydrochloric acid drop-wise until a faint turbidity develops, the pH of the solution is between 4.4 and 5.3.
Molecular weight determination: To pass the test.
the weight-average molecular weight MW = 34,000 to 60,000
and the number-average molecular weight MN = Not less than 24,000 Da
MW/MN = Not more than 1.7
The Uses of Iron Sucrose:
Iron sucrose injection is an iron replacement product that is used to treat iron deficiency anemia (not enough iron in the blood) in patients with chronic kidney disease. iT is used to treat "iron-poor" blood (anemia) in people with long-term kidney disease.
The MSDS-SDS Hazard Statement of Iron Sucrose:
Iron Sucrose SDS, Safety Data Sheet
MSDS Sheet, Material Safety Data Sheet 12-Jan-22
Section 1: Chemical Product and Identification
Product Name & Other Names: Iron Sucrose.
CAS#: 8047-67-4
EINECS EC-No.: 232-464-7
Relevant uses and uses advised against (if any): Laboratory and Industrial Manufacturing.
Section 2: Hazards Identification
GHS, Globally Harmonized System Classification in accordance with 29 CFR 1910
Classification according to Regulation (EC) No 1272/2008
Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008.
This substance is not classified as dangerous according to Directive 67/548/EEC.
Labeling according to GHS & Regulation (EC) No 1272/2008
GHS Label Elements NONE |
Signal Word: None
Hazards not otherwise classified (HNOC):
May causes mild skin irritation.
May Causes mild eye irritation and mechanical damage.
Precautionary statements:
P261: Avoid breathing dust/fume/gas/mist/vapors/spray.
P302+P352: IF ON SKIN: Wash with plenty of soap and water.
P304+P340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.
P337+313: If eye irritation persists get medical advice/attention.
Section 3: Composition and Information on Ingredients
Product Name & Other Names: Iron Sucrose.
CAS#: 8047-67-4
EINECS EC-No.: 232-464-7
Section 4: First Aid Measures
Always seek medical attention after first aid measures are provided.
Eye Contact: In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical attention.
Skin Contact: In case of contact, immediately flush skin with plenty of water. Cover the irritated skin with an emollient. Thoroughly clean shoes and clothes before reuse. Get medical attention.
Inhalation: If inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical attention.
Ingestion: Do NOT induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. If large quantities of this material are swallowed, call a physician immediately.
Section 5: Fire and Explosion Data
Flammability of the Product: Not applicable. Will burn at higher temperature.
Products of Combustion: Some metallic oxides, carbon oxides and fumes.
Explosion Hazards in Presence of Various Substances: Slightly explosive in presence of open flames and sparks. Non-explosive in presence of shocks. Fine dust dispersed in air in sufficient concentrations, and in the presence of an ignition source is a potential dust explosion hazard.
Fire Fighting Media and Instructions: Use water spray, alcohol-resistant foam, dry chemical, or carbon dioxide.
Special Information: In the event of a fire, wear full protective clothing and NIOSH-approved self-contained breathing apparatus with full face piece operated in the pressure demand or other positive pressure mode. At high temperatures under fire conditions, it may produce toxic or irritating fumes.
Section 6: Accidental Release Measures
Personal precautions, protective equipment, and emergency procedures: Avoid breathing dust/fumes/gas/mist/vapors/spray. Use individual protective equipment (waterproof boots, suitable protective clothing, safety glasses, etc.).
Environmental precautions: Do not let the product enter drains, soil or water sources.
Methods and materials used for containment Cleanup procedures and Storage: Use a shovel to put the material into a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and dispose as per law.
Section 7: Handling and Storage
Precautions for safe handling: Apply according to good manufacturing and industrial hygiene practices. Ensure proper ventilation. Wash thoroughly after handling. Do not drink, eat, or smoke while handling. Avoid contact with skin, eyes, and clothing. Minimize dust generation. Avoid breathing dust/fumes/gas/mist/vapors/spray. Keep container tightly closed. Avoid ingestion and inhalation. Use individual protective equipment (waterproof boots, suitable protective clothing, safety glasses, etc.).
Conditions for safe storage, including any incompatibilities: Store in cool, dry, and ventilated area away from heat sources and protected from sunlight in tightly closed original container. Keep air contact to a minimum. Do not leave the material container open. Store protected from heat, sparks and ignition sources and incompatible materials. Avoid contact with skin and eyes. Avoid inhalation of dust/mist/vapor. Do not store with incompatible materials like strong oxidizing agents.
Section 8: Exposure Controls/Personal Protection
Exposure Limits: This product does not contain any hazardous materials with occupational exposure limits established by the region-specific regulatory bodies.
Engineering Controls: Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended exposure limits.
Ventilation System: A system of local and/or general exhaust is recommended to keep employee exposures as low as possible.
Personal Respirators (NIOSH Approved): For conditions of use where exposure to dust or mist is apparent and engineering controls are not feasible, a particulate respirator may be worn.
Skin Protection: Wear protective gloves and clean body-covering clothing.
Eye Protection: Use chemical safety goggles and/or full face shield where dusting or splashing of solutions is possible. Maintain eye wash fountain and quick-drench facilities in work area.
Other Control Measures: Maintain good housekeeping in work area. Handle in accordance with good industrial hygiene and safety practice.
Section 9: Physical and Chemical Properties
Physical state and appearance: Iron Sucrose is reddish deep brown colored amorphous Powder, with characteristic odor and hygroscopic in nature. Solid.
Odor: Characteristic odor.
Odor threshold: Not available.
Taste: Sweet.
pH (2%w/v Iron Solution): 9.5 to 11.1
Relative density: Not available.
Melting Point: Not available.
Initial boiling point and boiling range: Not available.
Flash point: Not available.
Auto-ignition temperature: Not available.
Decomposition temperature: Not available.
Upper/lower flammability or explosive limits: Not available.
Vapor pressure: Not available.
Vapor density: Not available.
Evaporation rate: Not available.
Flammability (solid, gas): Not available.
Partition coefficient: n-octanol/water: Not available.
Solubility(ies): Not available.
Viscosity: Not available.
Section 10: Stability and Reactivity Data
Stability: It is stable.
Incompatible materials: Strong oxidizing agents
Incompatibility with various substances: Contact with incompatible materials. Heat, sparks, flames. Avoid dust formation.
Polymerization: Will not occur.
Hazardous Decomposition Products: Metallic oxides, carbon oxides and fumes.
Section 11: Toxicological Information
Toxicity to Animals: Not available.
Carcinogenicity: No component of this product present at levels greater than or equal to 0.1% is identified as probable or confirmed human carcinogen by IARC, ACGIH, OSHA and NTP.
Mutagenic Effects: Not available.
Environmental Toxicity: Not available.
Section 12: Ecological Information
Ecotoxicity: Not available.
Persistence and Degradability: Soluble in water Persistence is unlikely based on information available.
Mobility: Will likely be mobile in the environment due to its water solubility.
Results of PBT and vPvB assessment: Not available.
Section 13: Disposal Considerations
Waste Disposal: Waste must be disposed of in accordance legal regulations.
Section 14: Transport Information
DOT USA, TDG Canada & ADR/RID Europe: Not controlled.
IMO/IMDG: Not controlled.
IATA: Not controlled.
Section 15: Other Regulatory Information
USA:
SARA 311/312 Hazard Categories: Not applicable
California Proposition 65: This product does not contain any Proposition 65 chemicals.
Disclaimer:
**************************
Our company provides this MSDS sheet in good faith but makes no representation as to its comprehensiveness or accuracy. This SDS sheet is intended only as a guide to the appropriate precautionary handling of the material by a properly trained person using this product. The above information has been compiled from various sources and has the possibility of discrepancy and being out-dated information. Individuals receiving the information must exercise their independent judgment and do further search in determining its appropriateness for a particular purpose. In no case shall our company be liable to loss or damages by the product user.
**************************
Anmol Chemicals & Pharmaceuticals Pvt. Ltd. is an off-shoot of Anmol Chemicals Taloja. It is located in MIDC Taloja and it is manufacturing pharmaceutical grades of API, Excepients, Food grade and Reagent grade chemicals. Anmol Chemicals & Pharmaceuticals Pvt. Ltd. is a several decades old group of companies, engaged in manufacturing, supplying, distributing, wholesale supplies for actual users, retail or small pack supplies for research and development chemicals, fine and speciality chemicals, pharmaceutical excipients, mineral fortifiers in chemically pure, Analytical reagent grade, IP BP USP Ph Eur EP JP and other pharmaceutical grade monograph including FCC Food grade chemicals and Nutraceuticals, Mineral Fortifiers at best prices.
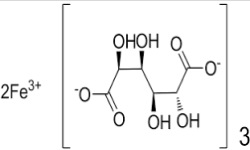
Iron Sucrose Structure
CAS Number 8047-67-4, Iron Sucrose Manufacturer Exporter
ANMOL CHEMICALS & PHARMACEUTICALS Pvt. Ltd.
India, USA, Europe, UAE
TELEPHONE: +912223770100
Navi Mumbai, INDIA
e-mail: info(At the rate i.e. @)anmol.org
Copyright. 18-nov-24
We manufacture:
Glacial Acetic Acid Manufacturer

