CAS Number 1310-66-3 monohydrate or 1310-65-2 anhydrous, Lithium Hydroxide Monohydrate Anhydrous USP Grade Manufacturers Exporters







CAS Number 1310-66-3 monohydrate or 1310-65-2 anhydrous, Lithium Hydroxide Monohydrate Anhydrous Manufacturer Exporter
For Properties Specifications of Lithium Hydroxide Monohydrate Anhydrous Click Properties, Specifications of Lithium Hydroxide Monohydrate Anhydrous Manufacturer.
For Uses of Lithium Hydroxide Monohydrate Anhydrous Click Uses of Lithium Hydroxide Monohydrate Anhydrous Manufacturer.
For For SDS MSDS Sheet of Lithium Hydroxide Monohydrate Anhydrous Click SDS Safety Data Sheet MSDS Sheet of Lithium Hydroxide Monohydrate Anhydrous Manufacturer.
The Properties and Specifications of Lithium Hydroxide Monohydrate Anhydrous:
Specifications of Lithium Hydroxide USP Grade:
LiOH-H2O --- 41.96
LiOH --- 23.95
Lithium hydroxide monohydrate CAS 1310-66-3.
Lithium hydroxide Anhydrous CAS 1310-65-2.
DEFINITION
Lithium Hydroxide contains NLT 98.0% and NMT 102.0% of lithium hydroxide (LiOH), calculated on the anhydrous basis.
IDENTIFICATION
A. When moistened with hydrochloric acid, it imparts an intense crimson color to a nonluminous flame.
Chloride and Sulfate, Sulfate:
Sample: 2.0 g
Acceptance criteria: It shows no more sulfate than corresponds to 1.0 mL of 0.020 N sulfuric acid (0.05%).
Calcium:
Sample solution: Dissolve 3.33 g in 50 mL of 3 N hydrochloric acid. Boil the clear solution to expel carbon dioxide, add 5 mL of ammonium oxalate, render alkaline with 6 N ammonium hydroxide, and allow to stand for 4 h. Pass through a filtering crucible, and wash with warm water until the last washing yields no turbidity with calcium chloride. Place the crucible in a beaker, cover it with water, add 3 mL of sulfuric acid, and heat to 70C.
Analysis: Titrate the Sample solution with 0.10 N potassium permanganate to a pale pink color that persists for 30 s.
Acceptance criteria: NMT 3.34 mL of 0.10 N potassium permanganate is consumed (0.20%).
Carbonate:
[Note: While pipeting and during the subsequent titrations, keep the contents of the flasks blanketed with a stream of carbon dioxide-free air.]
Analysis: To the flask containing the completed Final titration obtained in the ;Assay, add 1 drop of methyl orange TS. Titrate with 0.1 N hydrochloric acid VS until a persistent orange color is produced and no undissolved barium carbonate remains. Perform a blank titration to determine the volume of 0.1 N hydrochloric acid consumed in going from the phenolphthalein endpoint to the methyl orange endpoint. To 100 mL of carbon dioxide-free water in a 250-mL conical flask, add 3 drops of the Sample solution from the Assay, 20 mL of 1 N barium chloride, and 3 drops of phenolphthalein TS. Allow to stand for 2 min. Titrate this solution with 0.1 N hydrochloric acid. At the discharge of the pink color of the indicator, add 1 drop of methyl orange TS, and titrate with 0.1 N hydrochloric acid VS until a persistent orange color is produced.
Acceptance criteria: The titration shows no more carbon dioxide than corresponds to 1.5 mL of 0.10 N hydrochloric acid (0.7%).
Lithium Content: To pass the test by Flame photometry.
Acceptance criteria: 28.1% to 29.9% on the anhydrous basis.
Water Determination:
Analysis: Dry at 135C at a pressure of NMT 5 mm of mercury for 1 h.
Acceptance criteria: 41.0% to 43.5%
The Uses of Lithium Hydroxide Monohydrate Anhydrous:
Lithium Hydroxide is used in making lithium salts (soaps) of stearic and other fatty acids; these soaps are widely used as thickeners in lubricating greases. Lithium hydroxide is used to absorb unwanted gas. It is that it is used as an electrolyte in batteries. Nickel hydrogen batteries, nickel-cadmium batteries etc. It is often used to prevent corrosion. Submarines employ LiOH as a carbon dioxide scrubber. It is used as a catalyst in the production of alkyd resins in esterifications.
The MSDS-SDS Hazard Statement of Lithium Hydroxide Monohydrate Anhydrous:
Lithium Hydroxide Monohydrate and Anhydrous SDS, Safety Data Sheet
MSDS Sheet, Material Safety Data Sheet 12-Jan-23
Section 1: Product Identification
Product Name & Other Names: Lithium Hydroxide Monohydrate and Anhydrous.
CAS No.: 1310-66-3 monohydrate or 1310-65-2 anhydrous
EINECS EC Number: 215-183-4 or 603-454-3
Molecular Weight: 23.95 for anhydrous and 41.96 for Monohydrate
Chemical Formula: LiOH or anhydrous and LiOH-H2O for monohydrate
Relevant uses and uses advised against (if any) : Laboratory chemicals, Manufacture of substances. For Industrial use.
Section 2: Hazard Identification
GHS, Globally Harmonized System Classification in accordance with 29 CFR 1910
Classification according to Regulation (EC) No 1272/2008
Acute toxicity, oral Category 4, H302
Skin corrosion/irritation Category 1A, B, C, H314
Labeling according Regulation (EC) No 1272/2008
GHS Label Elements  Corrosive |
Signal Words: Danger
Hazard statements:
H302: Harmful if swallowed
H314: Causes severe skin burns and eye damage
Precautionary statements:
P260: Do not breathe dust/fume/gas/mist/vapors/spray.
P264: Wash … thoroughly after handling.
P270: Do not eat, drink or smoke when using this product.
P273: Avoid release to the environment.
P280: Wear protective gloves/protective clothing/eye protection/face protection.
P312: Call a POISON CENTER or doctor/physician if you feel unwell.
P362: Take off contaminated clothing and wash before reuse.
P301+330+331: IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
P301+312: IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell.
P303+361+353: IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse skin with water/shower.
P305+351+338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present and easy to do – continue rinsing.
Section 3: Composition / Information on Ingredients
Product Name & Other Names: Lithium Hydroxide Monohydrate and Anhydrous.
CAS No.: 1310-66-3 monohydrate or 1310-65-2 anhydrous
EINECS EC Number: 215-183-4 or 603-454-3
Section 4: First Aid Measures
Always seek medical attention after first aid measures are provided.
Eye Contact: Check for and remove any contact lenses. In case of contact, immediately flush eye with plenty of water for at least 15 minutes. Cold water may be used. Get medical attention immediately.
Skin Contact: Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Call a physician, immediately. Wash clothing before reuse.
Ingestion: If swallowed, do not induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to and unconscious person. Loosen tight clothing such as a collar, tie, belt, or waistband. Get medical attention immediately.
Medical Conditions generally Aggravated by Exposure: Repeated exposure of the eyes to a low level of dust can produce eye irritation. Repeated skin exposure can produce local skin destruction, or dermatitis. Repeated inhalation of dust can produce varying degree of respiratory irritation or lung damage.
Section 5: Fire Fighting Measures
Flash Point: NA
Fire Extinguishing Media: Adapt extinguishing media to the environment. Use water spray, alcohol-resistant foam, dry chemical, or carbon dioxide.
Special Fire Fighting Procedures: Wear self contained breathing apparatus for firefighting if necessary. Stay in danger area only with self-contained breathing apparatus. Prevent skin contact by keeping a safe distance or by wearing suitable protective clothing.
Unusual Fire and Explosion Hazards: Flammable hydrogen gas may be produced on prolong contact with metals such as aluminum, tin lead and zinc.
Hazardous combustion products: Oxides of Lithium.
Section 6: Accidental Release Measures
Personal precautions, protective equipment, and emergency procedures: Wear suitable protective equipment. Ventilate area of leak or spill. Avoid breathing dust/fumes/gas/mist/vapors/spray. Use individual protective equipment (waterproof boots, suitable protective clothing, safety glasses, etc.). Do not approach facing the wind.
Environmental precautions: Do not let the product enter drains, soil, or water sources.
Methods and materials used for containment Cleanup procedures and Storage: Small quantities of liquid spill may be neutralize with acid solution. Wash away neutralized product with plentiful water. Contain spilled material. Take up liquid spill into absorbent material, e.g.: dry sand/earth or powdered limestone. Cover with an inert, non-combustible absorbent material, (e.g. sand, earth, diatomaceous earth, vermiculite). Vacuum or sweep-up and remove to an approved disposal container.
Section 7: Handling and Storage
Precautions for safe handling: Apply according to good manufacturing and industrial hygiene practices. Ensure proper ventilation. Wash thoroughly after handling. Do not drink, eat, or smoke while handling. Avoid contact with skin, eyes, and clothing. Minimize dust generation. Avoid breathing dust/fumes/gas/mist/vapors/spray. Keep container tightly closed. Avoid ingestion and inhalation. Use individual protective equipment (waterproof boots, suitable protective clothing, safety glasses, etc.).
Conditions for safe storage, including any incompatibilities: Store in cool, dry, and ventilated area away from heat sources and protected from sunlight in tightly closed original container. Keep air contact to a minimum. Store protected from heat, sparks and ignition sources and incompatible materials. Avoid contact with skin and eyes. Avoid inhalation of dust/mist/vapor. Do not store with incompatible materials like strong oxidizing agents and water, acids, metals. Always add the material to water while stirring and never the reverse.
Section 8: Exposure Controls / Personal Protection
Components with workplace control parameters: This product does not contain any hazardous materials with occupational exposure limits established by the region specific regulatory bodies.
Engineering Controls Ventilation required: The material should be handled or transferred in an approved fume hood or with adequate ventilation.
Personal Protection Equipment
Respiratory protection: If workplace exposure limit (s) of product or any component is high, a NIOSH/MSHA approved air supplied respirator is advised in absence of proper environmental control.
Skin protection: Impervious, protective clothing. Protective gloves: Nitrile or equivalent.
Eye protection: Safety glasses with side shields must always be worn .
Additional clothing and/or equipment: Eyewash and safety equipment should be readily available. Handle in accordance with good industrial hygiene and safety practice.
Other Control Measures: Maintain good housekeeping in work area. Handle in accordance with good industrial hygiene and safety practice.
Section 9 Physical and Chemical Properties
Appearance: White Crystalline Powder. Hygroscopic.
Odor: Odorless.
Odor threshold: Not available.
pH: Not available.
Relative density: around 1.46 for anhydrous and around 1.51 for monohydrate.
Boiling Point: 924C.
Melting Point: 462C.
Flash point: Not available.
Auto-ignition temperature: Not available.
Decomposition temperature: Not available.
Upper/lower flammability or explosive limits: Not available.
Vapor pressure: Not available.
Vapor density: Not available.
Evaporation rate: Not available.
Flammability (solid, gas): Not available.
Partition coefficient: n-octanol/water: Not available.
Solubility: Soluble in water. Slightly soluble in ethanol.
Viscosity: Not available.
Section 10: Stability and Reactivity
Stability: Stable under ordinary conditions of use and storage. Very hygroscopic. Absorbs atmospheric CO2.
Reactivity: Exothermic reaction with water. Heat of solution is extremely high and with limited amounts of water, violent boiling may occur. Violent exothermic reaction with strong acids. Reacts with some metals to release hydrogen.
Conditions to Avoid: Contact with moisture may generate sufficient heat to ignite surrounding combustible material.
Materials to Avoid (Incompatibility): Water, acids, chlorinated hydrocarbons, oxidizing agents, metals and organic materials, Nitro compounds, Organic materials, Magnesium, Copper, Water, reacts violently with:, Metals, Light metals, Contact with aluminum, tin and zinc liberates hydrogen gas. Contact with nitromethane and other similar nitro compounds causes formation of shock-sensitive salts., vigorous reaction with: Alkali metals, Halogens, Azides, Anhydrides
Hazardous Decomposition Products: None indicated.
Hazardous Polymerization: Will not occur.
Section 11: Toxicological Information
The material is corrosive to all tissues. It is a severe eye, skin, and respiratory tract irritant, and can burn any tissue with which it comes in contact. Contact with eye may cause redness, intense pain, and tearing. In severe cases, conjunctival edema and destruction of cornea may occur, which may result in permanent damage to the eye. It is a severe skin irritant and contact with the skin can cause effects ranging from irritation to burns with deep and painful lesions. Burns may not be immediately painful. The onset of pain after contact may take minutes to hours; however, damage begins immediately.
Extremely hazardous in case of eye contact ( corrosive). Causes severe eye burns. Extremely hazardous in case of skin contact (corrosive). Skin contact produces severe burns. Hazardous in case of skin contact (permeator). Extremely hazardous in case of inhalation (lung corrosive). Hazardous in case of inhalation. Extremely hazardous in case of ingestion. May be fatal if swallowed. This product does not contain any compounds listed by NTP or IARC or regulated by OSHA as a carcinogen.
Results of component toxicity test performed:
LD50 Oral - Mouse - 363 mg/kg
LD50 Oral - Rat - 210 mg/kg
LC50 Inhalation - Rat - 4 h: > 615 mg/l
Carcinogenicity: This product is not classifiable as to its carcinogenicity based on its IARC, ACGIH, NTP, or EPA classification.
Section 12: Ecological Information
Toxicity to fish: static test LC50 Danio rerio (zebra fish): 62.2 mg/l; 96 h.
Toxicity to daphnia and other aquatic invertebrates: static test EC50 Daphnia magna (Water flea): 19.1 mg/l; 48 h.
Results of PBT and vPvB assessment:This substance/mixture contains no components considered to be either persistent, bioaccumulative and toxic (PBT), or very persistent and very bioaccumulative (vPvB) at levels of 0.1% or higher.
Expected to be highly toxic to aquatic organisms and ecosystems due to effects on pH. Harmful effect due to pH shift. Discharge into the environment must be avoided.
Section 13 Disposal Considerations
Offer surplus and non-recyclable solutions to a licensed disposal company. Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber.
Section 14: Transportation Information
DOT USA, TDG Canada & ADR/RID Europe:
Proper shipping name: LITHIUM HYDROXIDE
Hazard Class: 8
Packaging group: II
UN Number: UN2680
IATA:
Proper shipping name: LITHIUM HYDROXIDE
Hazard Class: 8
Packaging group: II
UN Number: UN2680
IMDG/IMO:
Proper shipping name: LITHIUM HYDROXIDE
Hazard Class: 8
Packaging group: II
UN Number: UN2680
Section 15: Regulatory Information
USA:
Sara 311/312: See section 2.
Section 16: Other Information
Disclaimer:
**************************
Our company provides this MSDS sheet in good faith but makes no representation as to its comprehensiveness or accuracy. This SDS sheet is intended only as a guide to the appropriate precautionary handling of the material by a properly trained person using this product. The above information has been compiled from various sources and has the possibility of discrepancy and being out-dated information. Individuals receiving the information must exercise their independent judgment and do further search in determining its appropriateness for a particular purpose. In no case shall our company be liable to loss or damages by the product user.
**************************
Anmol Chemicals & Pharmaceuticals Pvt. Ltd. is an off-shoot of Anmol Chemicals Taloja. It is located in MIDC Taloja and it is manufacturing pharmaceutical grades of API, Excepients, Food grade and Reagent grade chemicals. Anmol Chemicals & Pharmaceuticals Pvt. Ltd. is a several decades old group of companies, engaged in manufacturing, supplying, distributing, wholesale supplies for actual users, retail or small pack supplies for research and development chemicals, fine and speciality chemicals, pharmaceutical excipients, mineral fortifiers in chemically pure, Analytical reagent grade, IP BP USP Ph Eur EP JP and other pharmaceutical grade monograph including FCC Food grade chemicals and Nutraceuticals, Mineral Fortifiers at best prices.
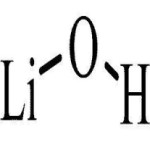
Lithium Hydroxide Monohydrate Anhydrous Structure
CAS Number 1310-66-3 monohydrate or 1310-65-2 anhydrous, Lithium Hydroxide Monohydrate Anhydrous Manufacturer Exporter
ANMOL CHEMICALS & PHARMACEUTICALS Pvt. Ltd.
India, USA, Europe, UAE
TELEPHONE: +912223770100
Navi Mumbai, INDIA
e-mail: info(At the rate i.e. @)anmol.org
Copyright. 19-nov-24
We manufacture:

