CAS Number 5949-29-1 & 77-92-9, Citric Acid Monohydrate Anhydrous USP BP Ph Eur Analytical Reagent FCC Food Grade Manufacturers Exporters







CAS Number 5949-29-1 & 77-92-9, Citric Acid Monohydrate Anhydrous Manufacturer Exporter
For Properties Specifications of Citric Acid Monohydrate Anhydrous Click Properties, Specifications of Citric Acid Monohydrate Anhydrous Manufacturer.
For Uses of Citric Acid Monohydrate Anhydrous Click Uses of Citric Acid Monohydrate Anhydrous Manufacturer.
For For SDS MSDS Sheet of Citric Acid Monohydrate Anhydrous Click SDS Safety Data Sheet MSDS Sheet of Citric Acid Monohydrate Anhydrous Manufacturer.
The Properties and Specifications of Citric Acid Monohydrate Anhydrous:
Citric Acid Monohydrate USP Grade Specifications
Citric Acid USP Monohydrate
C6H8O7·H2O 210.14
1,2,3-Propanetricarboxylic acid, 2-hydroxy-, monohydrate.
Citric Acid Monohydrate contains one molecule of water of hydration. It contains not less than 99.5 percent and not more than 100.5 percent of C6H8O7, calculated on the anhydrous basis.
Labeling: Where it is intended for use in dialysis solutions, it is so labeled. Where Citric Acid Monohydrate must be subjected to further processing during the preparation of injectable dosage forms to ensure acceptable levels of bacterial endotoxins, it is so labeled. Where Citric Acid Monohydrate is sterile, it is so labeled.
Identification, Infrared Absorption 197K: Dry the substance to be examined at 105 for 2 hours.
Bacterial endotoxins: The level of bacterial endotoxins is such that the requirement in the relevant dosage form monograph (s) in which Citric Acid Monohydrate is used can be met.
Sterility: Where the label states that Citric Acid Monohydrate is sterile, it meets the requirements for Sterility, in the relevant dosage form monograph(s) in which Citric Acid Monohydrate is used.
Water: between 7.5% and 9.0%.
Residue on ignition: Not more than 0.1%, determined on 1.0 g.
Readily carbonizable substances: To pass the test
Sulfate: 0.015%.
Heavy metals: 0.001%.
Limit of oxalic acid: 0.036%.
Citric Acid Monohydrate BP Ph Eur Grade Specifications
C6H8O7-H2O -- 210 -- CAS 5949-29-1
Citric Acid BP Monohydrate
DEFINITION
2-Hydroxypropane-1,2,3-tricarboxylic acid monohydrate.
Content: 99.5 per cent to 100.5 per cent (anhydrous substance).
CHARACTERS
Appearance: White or almost white, crystalline powder, colourless crystals or granules, efflorescent.
Solubility: Very soluble in water, freely soluble in ethanol (96 per cent).
IDENTIFICATION
First identification B, E.
Second identification A, C, D, E.
A. Dissolve 1 g in 10 ml of water. The solution is strongly acidic.
B. Infrared absorption spectrophotometry.
C. Add about 5 mg to a mixture of 1 ml of acetic anhydride and 3 ml of pyridine. A red colour develops.
D. Dissolve 0.5 g in 5 ml of water, neutralize using 1 M sodium hydroxide (about 7 ml), add 10 ml of calcium chloride solution R and heat to boiling. A white precipitate is formed.
E. Water (see Tests).
TESTS
Appearance of solution: The solution is clear and not more intensely coloured than reference.
Dissolve 2.0 g in water and dilute to 10 ml with the same solvent.
Readily carbonizable substances: To 1.0 g in a cleaned test tube add 10 ml of sulphuric acid and immediately heat the mixture in a water-bath at 90C ± 1C for 60 min. Cool rapidly immediately afterwards. The solution is not more intensely coloured than a mixture of 1 ml of red primary solution and 9 ml of yellow primary solution.
Oxalic acid: Maximum 360 ppm, calculated as anhydrous oxalic acid.
Sulphates: Maximum 150 ppm.
Aluminium: Maximum 0.2 ppm, if intended for use in the manufacture of dialysis solutions.
Heavy metals: Maximum 10 ppm.
Water: 7.5 per cent to 9.0 per cent, determined on 0.500 g.
Sulphated ash: Maximum 0.1 per cent, determined on 1.0 g.
Bacterial endotoxins: Less than 0.5 IU/mg, if intended for use in the manufacture of parenteral dosage forms without a further appropriate procedure for the removal of bacterial endotoxins.
We also manufacture Citric Acid IP EP Grade and Citric Acid Anhydrous USP Grade
Citric Acid FCC Food Grade SpecificationsC6H8O7 Formula weight, anhydrous 192.13
C6H8O7·H2O Formula weight, monohydrate 210.14
INS: 330 CAS: anhydrous CAS 77-92-9
CAS: monohydrate CAS 5949-29-1
DESCRIPTION
Citric Acid occurs as colorless, translucent crystals or as a white, granular to fine, crystalline powder. It is anhydrous or contains one molecule of water of hydration. The hydrous form is efflorescent in dry air. It is odorless and has a strongly acid taste. One gram is soluble in about 0.5 mL of water, in about 2 mL of alcohol, and in about 30 mL of ether.
Function: Sequestrant; dispersing agent; acidifier; flavoring agent.
REQUIREMENTS
Labeling: Indicate whether it is anhydrous or hydrous.
Identification: A 1:10 aqueous solution gives positive tests for Citrate.
Assay: Not less than 99.5% and not more than 100.5% of C6H8O7, calculated on the anhydrous basis.
Lead: Not more than 0.5 mg/kg.
Oxalate: Passes test.
Readily Carbonizable: Substances Passes test.
Residue on Ignition: Not more than 0.05%.
Tridodecylamine (for solvent-extracted Citric Acid only)
Not more than 0.1 mg/kg.
Water: Anhydrous: Not more than 0.5%; Monohydrate: Not more than 8.8%.
Citric Acid Analytical Reagent
Citric Acid, Anhydrous, and Citric Acid, Monohydrate
2-Hydroxy-1,2,3-propanetricarboxylic Acid
HOCOCH2C(OH)(COOH)CH2COOH; Formula Weight. 192.13
HOCOCH2C(OH)(COOH)CH2COOH-H2O; Formula Weight. 210.14
CAS Number 77-92-9 Anhydrous and 5949-29-1 Monohydrate
REQUIREMENTS
Assay: 99.5% C6H8O7; 99.0-102.0% C6H8O7-H2O min
MAXIMUM ALLOWABLE
Insoluble matter: 0.005%
Residue after ignition: 0.02%
Chloride (Cl): 0.001%
Oxalate (C2O4): Passes test
Phosphate (PO4): 0.001%
Sulfate (SO4): 0.002%
Iron (Fe): 3 ppm
Lead (Pb): 2 ppm
Substances carbonizable by hot sulfuric acid: Passes test.
The Uses of Citric Acid Monohydrate Anhydrous:
Citric acid is often added to packaged food and drinks. Citric acid can balance out the acid in a food or drink. Citric acid is used in food as a flavouring agent and preservative. It is used in Medicines, Supplements, Personal care products, Household cleaners, Disinfectants, Environmental cleanup products. Citric acid is a naturally occurring antioxidant.
The MSDS-SDS Hazard Statement of Citric Acid Monohydrate Anhydrous:
Citric Acid SDS MSDS Sheet, Material Safety Data Sheet 10-Jan-23
Section 1 - Chemical Product and Company Identification
MSDS Name: Citric Acid, Anhydrous
Synonyms: 2-Hydroxy-1,2,3-propanetricarboxylic acid
Intended Use: Oil well fracturing and Industrial use.
Section 2 - Hazards Identification
GHS, Globally Harmonized System Classification in accordance with 29 CFR 1910
Classification according to Regulation (EC) No 1272/2008
Acute toxicity, Dermal (Category 5)
Skin irritation (Category 3)
Eye irritation (Category 2A)
Labeling according GHS USA & Regulation (EC) No 1272/2008
GHS Label Elements  Irritant |
Signal Words: Warning
Hazard statements:
H313: May be harmful in contact with skin.
H316: Causes mild skin irritation
H319: Causes serious eye irritation
Precautionary statements:
P262: Do not get in eyes, on skin, or on clothing.
P264: Wash skin thoroughly after handling.
P280: Wear protective gloves/protective clothing/eye protection/face protection.
P270: Do not eat, drink or smoke when using this product.
P273: Avoid release to the environment.
P312: Call a POISON CENTER or doctor/physician if you feel unwell.
P332+313: If skin irritation occurs: Get medical advice/attention.
P302+ P352: IF ON SKIN: Wash with plenty of soap and water.
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.
P337+P313 If eye irritation persists: Get medical advice/ attention.
Section 3 - Composition, Information on Ingredients
Chemical Name: Citric acid
CAS#: 77-92-9
EINECS EC Number: 201-069-1
Section 4 - First Aid Measures
Always seek medical attention after first aid measures are provided.
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid. Do NOT allow victim to rub or keep eyes closed.
Skin: Flush skin with plenty of soap and water for at least 15 minutes while removing contaminated clothing and shoes. Get medical aid if irritation develops or persists. Wash clothing before reuse.
Ingestion: Do NOT induce vomiting. If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation: Remove from exposure to fresh air immediately. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Notes to Physician: Treat symptomatically and supportively.
Section 5 - Fire Fighting Measures
General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media: Use water spray, dry chemical, carbon dioxide, or chemical foam. Use agent most appropriate to extinguish fire. Do NOT get water inside containers.
Section 6 - Accidental Release Measures
General Information: Use proper personal protective equipment.
Spills/Leaks: Vacuum or sweep up material and place into a suitable disposal container. Very fine particles of Citric acid can cause a fire or explosion. Eliminate all ignition sources. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions. Remove all sources of ignition. Provide ventilation. Spill of Citric acid may be neutralized with lime. Do not get water inside containers.
Section 7 - Handling and Storage
Handling: Wash thoroughly after handling. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container of Citric acid tightly closed. Avoid ingestion and inhalation. Do not allow contact with water. Keep Citric acid from contact with moist air and steam.
Storage: Store Citric acid in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Store Citric acid protected from moisture.
Section 8 - Exposure Controls, Personal Protection
Airborne Exposure Limits: None established.
Ventilation System: A system of local and/or general exhaust is recommended to keep employee exposures as low as possible.
Personal Respirators (NIOSH Approved): For conditions of use where exposure to dust or mist is apparent and engineering controls are not feasible, a particulate respirator (NIOSH type N95 or better filters) may be worn.
Skin Protection: Wear impervious protective clothing, including boots, gloves, lab coat, apron or coveralls, as appropriate, to prevent skin contact.
Eye Protection: Use chemical safety goggles and/or full face shield where dusting or splashing of solutions is possible. Maintain eye wash fountain and quick-drench facilities in work area of Citric acid.
Section 9 - Physical and Chemical Properties
Appearance: Citric acid is white granules.
Odor: Citric acid is odorless.
Solubility: ca. 60 g/100 ml @ 20C (Anhydrous)
Density: 1.542
pH: 2.2 (0.1 N sol)
% Volatiles by volume @ 21C (70F): 0
Boiling Point: No information found.
Melting Point: ca. 100C (ca. 212F)
Section 10 - Stability and Reactivity
Chemical Stability: Citric acid is stable under normal temperatures and pressures.
Conditions to Avoid: Incompatible materials, dust generation, moisture, exposure to moist air or water.
Incompatibilities with Other Materials: Oxidizing agents, sulfides (inorganic, e.g. ferric sulfide, lead sulfide, sodium sulfide), metal nitrates, alkali carbonates, alkalis, potassium tartrate, acetates, bicarbonates.
Hazardous Decomposition Products: Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Will not occur.
Section 11 - Toxicological Information
Oral rat LD50: 3 gm/kg; irritation skin rabbit: 500 mg/24H mild; eye rabbit: 750 µg/24H severe.
Carcinogenicity: No component of this product present at levels greater than or equal to 0.1% is identified as probable, possible or confirmed human carcinogen by IARC, ACGIH, OSHA and NTP.
Section 12 - Ecological Information
Environmental Fate: No information found.
Environmental Toxicity: No information found.
Section 13 - Disposal Considerations
Whatever cannot be saved for recovery or recycling should be managed in an appropriate and approved waste disposal facility.
Section 14 - Transport Information
DOT: Not regulated as controlled.
ADR/RID: Not controlled.
IMDG: Not controlled.
IATA: Not controlled.
Section 15 - Regulatory Information
USA:
Section 302 (RQ): None of the chemicals in this material have an RQ.
Section 302 (TPQ): None of the chemicals in this product have a TPQ.
SARA Codes: CAS # 77-92-9: acute. CAS# 77-92-9: 0
Section 16 - Additional Information
DISCLAIMER: The information and recommendations set forth herein are presented in good faith and believed correct as of the date hereof. It is compiled from various sources and it is not necessarily all inclusive nor fully adequate in every circumstance. In addition, these suggestions should not be confused with nor followed in violation of applicable laws, regulations, rules or insurance requirements applicable. This MSDS sheet is intended only as a guide to the appropriate precautionary handling of the material by a properly trained person using this product. Individuals receiving the information must exercise their independent judgment in determining its appropriateness for a particular purpose.
Anmol Chemicals & Pharmaceuticals Pvt. Ltd. is an off-shoot of Anmol Chemicals Taloja. It is located in MIDC Taloja and it is manufacturing pharmaceutical grades of API, Excepients, Food grade and Reagent grade chemicals. Anmol Chemicals & Pharmaceuticals Pvt. Ltd. is a several decades old group of companies, engaged in manufacturing, supplying, distributing, wholesale supplies for actual users, retail or small pack supplies for research and development chemicals, fine and speciality chemicals, pharmaceutical excipients, mineral fortifiers in chemically pure, Analytical reagent grade, IP BP USP Ph Eur EP JP and other pharmaceutical grade monograph including FCC Food grade chemicals and Nutraceuticals, Mineral Fortifiers at best prices.
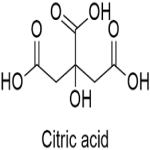
Citric Acid Monohydrate Anhydrous Structure
CAS Number 5949-29-1 & 77-92-9, Citric Acid Monohydrate Anhydrous Manufacturer Exporter
ANMOL CHEMICALS & PHARMACEUTICALS Pvt. Ltd.
India, USA, Europe, UAE
TELEPHONE: +912223770100
Navi Mumbai, INDIA
e-mail: info(At the rate i.e. @)anmol.org
Copyright. 9-jul-25
We manufacture:
Glacial Acetic Acid Manufacturer
Copper Sulfate or Cupric Sulphate

