CAS Number 12511-31-8 or 12174-11-7 or or 1327-43-1 or 12199-37-0, Aluminum Magnesium Silicate or Magnesium Aluminium Silicate USP NF BP Ph Eur Grade Manufacturers Exporters







CAS Number 12511-31-8 or 12174-11-7 or or 1327-43-1 or 12199-37-0, Aluminum Magnesium Silicate or Magnesium Aluminium Silicate Manufacturer Exporter
For Properties Specifications of Aluminum Magnesium Silicate or Magnesium Aluminium Silicate Click Properties, Specifications of Aluminum Magnesium Silicate or Magnesium Aluminium Silicate Manufacturer.
For Uses of Aluminum Magnesium Silicate or Magnesium Aluminium Silicate Click Uses of Aluminum Magnesium Silicate or Magnesium Aluminium Silicate Manufacturer.
For For SDS MSDS Sheet of Aluminum Magnesium Silicate or Magnesium Aluminium Silicate Click SDS Safety Data Sheet MSDS Sheet of Aluminum Magnesium Silicate or Magnesium Aluminium Silicate Manufacturer.
The Properties and Specifications of Aluminum Magnesium Silicate or Magnesium Aluminium Silicate:
Magnesium Aluminum Silicate USP NF Grade Specifications
Magnesium Aluminum Silicate is a blend of colloidal montmorillonite and saponite that has been processed to remove grit and non-swellable ore components.
The requirements for viscosity and ratio of aluminum content to magnesium content differ for the several types of Magnesium Aluminum Silicate, as set forth in the accompanying table.
| Type | Viscosity(cps) | (Al content)/(Mg content) |
| Min. ---- Max. | Min. ---- Max. | |
| IA | 0225 ---- 0600 | 0.5 ------ 1.2 |
| IB | 0150 ---- 0450 | 0.5 ------ 1.2 |
| IC | 0800 ---- 2200 | 0.5 ------ 1.2 |
| IIA | 0100 ---- 0300 | 1.4 ------ 2.8 |
Identification: Add 2 g in small portions to 100 mL of water, with intense agitation. Allow to stand for 12 hours to ensure complete hydration. Place 2 mL of the resulting mixture on a suitable glass slide, and allow to air-dry at room temperature to produce an oriented film. Place the slide in a vacuum desiccator over a free surface of ethylene glycol. Evacuate the desiccator, and close the stopcock so that the ethylene glycol saturates the desiccator chamber. Allow to stand for 12 hours. Record the Xray diffraction pattern, and calculate the d values: the largest peak corresponds to a d value between 15.0 and 17.2 angstrom units. Prepare a random powder specimen of Magnesium Aluminum Silicate, record the X-ray diffraction pattern, and determine the d values in the region between 1.48 and 1.54 angstrom units: peaks are found between 1.492 and 1.504 angstrom units and between 1.510 and 1.540 angstrom units.
Viscosity: After determining the Loss on drying, weigh a quantity of Magnesium Aluminum Silicate test specimen equivalent to 25.0 g on the dried basis. Over a period of a few seconds, transfer the un-dried test specimen to a suitable 1-L blender jar containing an amount of water, maintained at a temperature of 25 ± 2 , that is sufficient to produce a mixture weighing 500 g. Blend for 3 minutes, accurately timed, at 14,000 to 15,000 rpm (high speed). [NOTE—Heat generated during blending causes a temperature rise to above 30 .] Transfer the contents of the blender to a 600-mL beaker, allow to stand for 5 minutes, and adjust, if necessary, to a temperature of 33 ± 3. Using a suitable rotational viscosimeter equipped with a spindle as specified below, operate the viscosimeter at 60 rpm for 6 minutes, accurately timed, and record the scale reading. For Type IA, use a spindle having a cylinder 1.87 cm in diameter and 0.69 cm high attached to a shaft 0.32 cm in diameter, the distance from the top of the cylinder to the lower tip of the shaft being 2.54 cm, and the immersion depth being 5.00 cm (No. 2 spindle); if the scale reading is greater than 90% of full-scale, repeat the measurement, using a spindle similar to the No. 2 spindle but having the cylinder 1.27 cm in diameter and 0.16 cm high instead (No. 3 spindle). For Type IC, use a No. 3 spindle; if the scale reading is greater than 90% of full-scale, repeat the measurement using a spindle consisting of a cylindrical shaft 0.32 cm in diameter and having an immersion depth of 4.05 cm (No. 4 spindle). For Types IB and IIA, use a No. 2 spindle.
Microbial limits: Its total aerobic microbial count does not exceed 1000 cfu per g, and it meets the requirements of the test for absence of Escherichia coli.
pH: between 9.0 and 10.0, in a suspension (5 in 100) in water.
Loss on drying: Dry it at 110C to constant weight: it loses not more than 8.0% of its weight.
Acid demand: After determining the Loss on drying, weigh a quantity of Magnesium Aluminum Silicate equivalent to 5.00 g, and disperse in 500 mL of water with the aid of a suitable blender fitted with a 1-liter jar. Using a stopwatch, designate zero time. With constant mixing, add 3.0-mL portions of 0.100 N hydrochloric acid at 5, 65, 125, 185, 245, 305, 365, 425, 485, 545, 605, 665, and 725 seconds, and add a 1.0-mL portion at 785 seconds. Determine the pH potentiometrically at 840 seconds: the pH is not more than 4.0.
Arsenic: The limit is 3 ppm.
Lead: To pass the test The absorbance of the Test preparation is not greater than that of the Standard preparation (0.0015%).
Aluminium Magnesium Silicate BP Ph Eur Grade Specifications
Ph Eur
CAS 12511-31-8
Action and use: Excipient.
DEFINITION
Mixture of particles with colloidal particle size of montmorillonite and saponite, free from grit and non-swellable ore.
Content:
aluminium (Al; Ar 26.98): 95.0 per cent to 105.0 per cent of the value stated on the label.
magnesium (Mg; Ar 24.30): 95.0 per cent to 105.0 per cent of the value stated on the label.
CHARACTERS
Appearance: Almost white powder, granules or plates.
Solubility: Practically insoluble in water and in organic solvents. It swells in water to produce a colloidal dispersion.
IDENTIFICATION
A. Fuse 1 g with 2 g of anhydrous sodium carbonate. Warm the residue with water and filter. Acidify the filtrate with hydrochloric acid and evaporate to dryness on a water-bath. 0.25 g of the residue gives the reaction of silicates.
B. Dissolve the remainder of the residue obtained in identification test A in a mixture of 5 mL of dilute hydrochloric acid and 10 mL of water. Filter and add ammonium chloride buffer solution pH 10.0. A white, gelatinous precipitate is formed. Centrifuge and keep the supernatant for identification C. Dissolve the remaining precipitate in dilute hydrochloric acid. The solution gives the reaction of aluminium.
C. The supernatant liquid obtained after centrifugation in identification test B gives the reaction of magnesium.
TESTS
pH: 9.0 to 10.0.
Arsenic: Maximum 3 ppm.
Lead: Maximum 15 ppm.
Loss on drying: Maximum 8.0 per cent, determined on 1.000 g by drying in an oven at 105C.
Microbial contamination:
TAMC: acceptance criterion 1000 CFU/g.
TYMC: acceptance criterion 100 CFU/g.
Absence of Escherichia coli.
The Uses of Aluminum Magnesium Silicate or Magnesium Aluminium Silicate:
Aluminum Magnesium Silicate or Magnesium Aluminium Silicate is used as a pharmaceutical excipient. Magnesium aluminum silicate is used as an over the counter antacid for the self-treatment of heartburn, sour stomach, or acid indigestion. Magnesium silicate is used to absorb moisture, prevent caking, and to improve the feel of a product. In the pharmaceutical companies, it is used as a dietary supplement, as part of the formulation ingredients in drug production, in antacid and antiulcer preparations, as a component of antiepileptic drugs, in antifungal topical agents and in the treatment of acne and as a facial moisturizer. When complexed with arginine, magnesium silicate can be used as an antiatherosclerotic agent and to promote bone and cartilage formation in mammals.
The MSDS-SDS Hazard Statement of Aluminum Magnesium Silicate or Magnesium Aluminium Silicate:
Aluminum Magnesium Silicate SDS, Safety Data Sheet
MSDS Sheet, Material Safety Data Sheet 02-Jan-22
Section 1: Chemical Product and Company Identification
Product Name & Other Names: Aluminum Magnesium Silicate or Magnesium Aluminum Silicate.
CAS Number: 12511-31-8 or 12174-11-7 (also 1327-43-1 or 12199-37-0).
EINECS EC Code: 215-478-8 or 308-996-1
Chemical Formula: AlMgO4Si
Molecular Weight: 143.37
Relevant uses and uses advised against (if any): Industrial Manufacturing.
Section 2: Hazards Identification
GHS, Globally Harmonized System Classification in accordance with 29 CFR 1910
Classification according to Regulation (EC) No 1272/2008
Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008.
This substance is not classified as dangerous according to Directive 67/548/EEC.
Labeling according GHS & Regulation (EC) No 1272/2008
GHS Label Elements NONE |
Signal Word: None
Precautionary statements:
P261: Avoid breathing dust/fume/gas/mist/vapors/spray.
P262: Do not get in eyes, on skin, or on clothing.
P281: Use personal protective equipment as required.
P302+P352: IF ON SKIN: Wash with plenty of soap and water.
P303+P361+P353: IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse skin with water/shower.
P304+P340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.
Section 3: Composition and Information on Ingredients
Product Name & Other Names: Aluminum Magnesium Silicate or Magnesium Aluminum Silicate.
CAS Number: 12511-31-8 or 12174-11-7 (also 1327-43-1 or 12199-37-0).
EINECS EC Code: 215-478-8 or 308-996-1
Section 4: First Aid Measures
Always seek medical advice after the first aid treatment.
Skin: Rinse with water. Soap may be used. Seek Medical Aid.
Eyes: Wash eyes with plenty of water for at least 15 minutes, lifting lids occasionally. Seek Medical Aid.
Inhalation: Remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Ingestion: If swallowed, induce vomiting immediately after giving two glasses of water. Never give anything by mouth to an unconscious person.
Notes to Physician: Treat symptomatically.
Section 5: Fire and Explosion Data
Flammability of the Product: Non-flammable.
Products of Combustion: Aluminum oxide, Magnesium oxide, Silicon oxides.
Fire Fighting Media and Instructions: Use extinguishing measures that are appropriate to local circumstances and the surrounding environment.
Section 6: Accidental Release Measures
Personal precautions, protective equipment and emergency procedures: Avoid breathing dust/fumes/gas/mist/vapors/spray. Use individual protective equipment (waterproof boots, suitable protective clothing, safety glasses, etc.). Restrict unprotected personnel from the area. Do not approach facing the wind.
Environmental precautions: Do not let the product enter drains, soil or water sources.
Methods and materials used for containment Cleanup procedures and Storage: Contain spilled material. Do not inhale vapors, dust, mist or gas. Avoid dust formation. Use a shovel to put the material into a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and allow to evacuate as per law.
Section 7: Handling and Storage
Precautions for safe handling: Apply according to good manufacturing and industrial hygiene practices. Ensure proper ventilation. Wash thoroughly after handling. Do not drink, eat or smoke while handling. Avoid contact with skin, eyes and clothing. Minimize dust generation. Avoid breathing dust/fumes/gas/mist/vapors/spray. Keep container tightly closed. Avoid ingestion and inhalation. Use individual protective equipment (waterproof boots, suitable protective clothing, safety glasses, etc.).
Conditions for safe storage, including any incompatibilities: Store in cool, dry and ventilated area away from heat sources and protected from sunlight in tightly closed original container. Keep air contact to a minimum. Do not leave the material container open. Store protected from heat, sparks and ignition sources and incompatible materials. Avoid inhalation of dust/mist/vapor. Do not store with incompatible materials like oxidizing agents, acids.
Section 8: Exposure Controls/Personal Protection
Engineering Controls: Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants below the exposure limit.
Personal Protection: Safety glasses. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent Gloves.
Personal Protection in Case of a Large Spill: Splash goggles. Full suit. Dust respirator. Boots. Gloves. A self-contained breathing apparatus should be used to avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this product.
Section 9: Physical and Chemical Properties
Physical state and appearance: Solid white to off-white powder or granules.
Odor: Odorless.
Odor threshold: Not available.
pH: Not available.
Specific Gravity: 2.6
Melting point/freezing point: Not available.
Initial boiling point and boiling range: Not available.
Flash point: Not available.
Auto-ignition temperature: Not available.
Decomposition temperature: Not available.
Upper/lower flammability or explosive limits: Not available.
Vapor pressure: Not available.
Vapor density: Not available.
Evaporation rate: Not available.
Flammability (solid, gas): Not available.
Partition coefficient: n-octanol/water: Not available.
Solubility: Insoluble in cold water.
Viscosity: Not available.
Section 10: Stability and Reactivity Data
Stability: It is stable in room temperature in closed containers under normal storage & handling.
Conditions of instability: Incompatible materials
Incompatibility with various substances: Strong oxidizing agents.
Polymerization: Will not occur.
Section 11: Toxicological Information
Toxicity to Animals: LD50/oral/rat = > 16000 mg/kg Oral LD50 Rat.
Carcinogenic Effects: Not a reported carcinogen by IARC, NTP, ACGIH, OSHA.
Reproductive Effects: No information available
Developmental Effects: No information available.
Section 12: Ecological Information
Ecotoxicity: No specific data or information available regarding environmental impact.
Persistence and Degradability: No information available.
Mobility: No information available.
Bioaccumulation/ Accumulation: No information available.
Results of PBT and vPvB assessment: No data available for assessment.
Section 13: Disposal Considerations
Waste Disposal: Waste must be disposed of in accordance with federal, state and local environmental control regulations.
Section 14: Transport Information
DOT (USA): Not controlled.
IMDG: Not controlled.
IATA: Not controlled.
ADR/RID: Not controlled.
Section 15: Other Regulatory Information
USA Federal and State Regulations:
California Prop. 65 Components: This product does not contain any chemicals known to State of California to cause cancer, birth defects, or any other reproductive harm.
Section 16 - Additional Information
Disclaimer:
**************************
Our company provides this MSDS sheet in good faith but makes no representation as to its comprehensiveness or accuracy. This SDS sheet is intended only as a guide to the appropriate precautionary handling of the material by a properly trained person using this product. The above information has been compiled from various sources and has the possibility of discrepancy and being out-dated information. Individuals receiving the information must exercise their independent judgment and do further search in determining its appropriateness for a particular purpose. In no case shall our company be liable to loss or damages by the product user.
**************************
Anmol Chemicals & Pharmaceuticals Pvt. Ltd. is an off-shoot of Anmol Chemicals Taloja. It is located in MIDC Taloja and it is manufacturing pharmaceutical grades of API, Excepients, Food grade and Reagent grade chemicals. Anmol Chemicals & Pharmaceuticals Pvt. Ltd. is a several decades old group of companies, engaged in manufacturing, supplying, distributing, wholesale supplies for actual users, retail or small pack supplies for research and development chemicals, fine and speciality chemicals, pharmaceutical excipients, mineral fortifiers in chemically pure, Analytical reagent grade, IP BP USP Ph Eur EP JP and other pharmaceutical grade monograph including FCC Food grade chemicals and Nutraceuticals, Mineral Fortifiers at best prices.
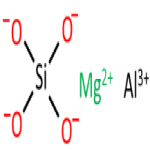
Aluminum Magnesium Silicate or Magnesium Aluminium Silicate
Structure
CAS Number 12511-31-8 or 12174-11-7 or or 1327-43-1 or 12199-37-0, Aluminum Magnesium Silicate or Magnesium Aluminium Silicate Manufacturer Exporter
ANMOL CHEMICALS & PHARMACEUTICALS Pvt. Ltd.
India, USA, Europe, UAE
TELEPHONE: +912223770100
Navi Mumbai, INDIA
e-mail: info(At the rate i.e. @)anmol.org
Copyright. 11-dec-24
We manufacture:
Glacial Acetic Acid Manufacturer
Aluminum Chloride Hexahydrate Anhydrous
Aluminum Chlorohydrate Solution Powder
Aluminium Sodium Silicate or Sodium Aluminosilicate
Dihydroxyaluminum Aminoacetate
Dihydroxyaluminum Sodium Carbonate

