CAS Number 298-14-6, Potassium Bicarbonate USP BP Ph Eur Analytical Reagent FCC Food Grade Manufacturers Exporters







CAS Number 298-14-6, Potassium Bicarbonate Manufacturer Exporter
For Properties Specifications of Potassium Bicarbonate Click Properties, Specifications of Potassium Bicarbonate Manufacturer.
For Uses of Potassium Bicarbonate Click Uses of Potassium Bicarbonate Manufacturer.
For For SDS MSDS Sheet of Potassium Bicarbonate Click SDS Safety Data Sheet MSDS Sheet of Potassium Bicarbonate Manufacturer.
The Properties and Specifications of Potassium Bicarbonate:
Potassium Bicarbonate USP Grade Specifications
KHCO3 100.12
Carbonic acid, monopotassium salt.
Monopotassium carbonate CAS 298-14-6
Potassium Bicarbonate contains not less than 99.5 percent and not more than 101.5 percent of KHCO3, calculated on the dried basis.
Identification: A solution (1 in 10) responds to the tests for Potassium and for Bicarbonate .
Loss on drying: Dry it over silica gel for 4 hours: it loses not more than 0.3% of its weight.
Normal carbonate: Grind 3.0 g of Potassium Bicarbonate with 25 mL of alcohol and 5 mL of water in a porcelain mortar. Add 3 drops of PhPh TS, and titrate slowly with barium chloride solution, prepared by dissolving 12.216 g of barium chloride in 300 mL of water and diluting with alcohol to obtain 1000 mL of solution, until the suspension becomes colorless. Continue the grinding for 2 minutes, and if the color turns pink, continue the titration with the barium chloride solution to a colorless end-point. Repeat the grinding for 2 minutes and the addition of the barium chloride solution, if necessary, until the suspension is colorless after 2 minutes of grinding. Each mL of the barium chloride solution is equivalent to 6.911 mg of K2CO3: not more than 2.5% is found.
Heavy metals: To 2 g add 5 mL of water and 8 mL of 3 N hydrochloric acid, heat to boiling, and maintain that temperature for 1 minute. Add 1 drop of PhPh and sufficient 6 N ammonium hydroxide, drop wise, to give the solution a faint pink color. Cool, add 2 mL of 1 N acetic acid, and then dilute with water to 25 mL: the limit is 0.001%.
Potassium Bicarbonate BP Ph Eur Grade specifications
Potassium, Hydrogen Carbonate, Ph Eur
KHCO3 100.1 CAS 298-14-6
DEFINITION
Content: 99.0 per cent to 101.0 per cent.
CHARACTERS
Appearance: White or almost white, crystalline powder or colourless crystals.
Solubility: Freely soluble in water, practically insoluble in ethanol (96 per cent).
When heated in the dry state or in solution, it is gradually converted to potassium carbonate.
IDENTIFICATION
A. To 5 mL of solution S (see Tests) add 0.1 mL of PhPh solution. A pale pink
colour is produced. Heat; gas is evolved and the colour becomes red.
B. It gives the reaction of carbonates and bicarbonates.
C. 1 mL of solution S gives reaction (b) of potassium.
TESTS
Solution S: Dissolve 5.0 g in 90 mL of carbon dioxide-free water prepared from distilled water and dilute to 100 mL with the same solvent.
Appearance of solution: Solution S is clear and colourless.
Carbonates: The pH of freshly prepared solution S is not greater than 8.6.
Chlorides: Maximum 150 ppm.
Sulfates: Maximum 150 ppm.
Ammonium: Maximum 20 ppm.
Calcium: Maximum 100 ppm.
Iron: Maximum 20 ppm, determined on solution S.
Sodium: Maximum 0.5 per cent.
Atomic emission spectrometry: To pass the test.
Heavy metals: Maximum 10 ppm.
Potassium Bicarbonate FCC Food Grade Specifications
KHCO3 Formula weight 100.12
INS: 501(ii) CAS 298-14-6
DESCRIPTION
Potassium Bicarbonate occurs as colorless, transparent, monoclinic prisms or as a white, granular powder. It is stable in air. Its solutions are neutral or alkaline to PhPh. One gram dissolves in 2.8 mL of water. It is almost insoluble in alcohol.
Function: pH control; leavening agent.
REQUIREMENTS
Identification A 1:10 aqueous solution gives positive tests for Potassium and for Bicarbonate.
Assay: Not less than 99.0% and not more than 101.5% of KHCO3, calculated on the dried basis.
Carbonate: Passes test.
Lead: Not more than 2 mg/kg.
Loss on Drying: Not more than 0.25%.
Potassium Bicarbonate Analytical Reagent Grade Specifications
Potassium Hydrogen Carbonate
KHCO3
Formula Weight: 100.12
CAS Number: 298-14-6
REQUIREMENTS
Assay (dried basis): 99.7-100.5% KHCO3
MAXIMUM ALLOWABLE
Insoluble matter: 0.01%
Chloride (Cl): 0.001%
Phosphate (PO4): 5 ppm
Sulfur compounds (as SO4): 0.003%
Ammonium (NH4): 5 ppm
Heavy metals (as Pb): 5 ppm
Iron (Fe): 5 ppm
Calcium (Ca): 0.002%
Magnesium (Mg): 0.001%
Sodium (Na): 0.03%.
The Uses of Potassium Bicarbonate:
Potassium bicarbonate is used as an antacid, electrolyte replenisher and potassium supplement. It can also be used as an excipient in drug formulations. It is a mineral supplement used to treat or prevent low amounts of potassium in the blood.
The MSDS-SDS Hazard Statement of Potassium Bicarbonate:
Potassium Bicarbonate SDS GHS, MSDS Sheet, Safety Data Sheet
1. PRODUCT DESCRIPTION
Product Name: Potassium Bicarbonate
CAS Number: 298-14-6
EINECS EC Number: 206-059-0
Recommended usage: Industrial Manufacturing.
2. HAZARD IDENTIFICATION
GHS, Globally Harmonized System Classification in accordance with 29 CFR 1910
Classification according to Regulation (EC) No 1272/2008
Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008.
This substance is not classified as dangerous according to Directive 67/548/EEC.
Labeling according to GHS & Regulation (EC) No 1272/2008
GHS Label Elements None |
Signal Words: None
Precautionary statements:
P261: Avoid breathing dust/fume/gas/mist/vapors/spray.
P262: Do not get in eyes, on skin, or on clothing.
P281: Use personal protective equipment as required.
P302+P352: IF ON SKIN: Wash with plenty of soap and water.
P304 + P340 - IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.
P337+313: If eye irritation persists get medical advice/attention.
Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008.
This substance is not classified as dangerous according to Directive 67/548/EEC.
3. COMPOSITION/INFORMATION ON INGREDIENTS
Principal Components: Potassium bicarbonate
CAS Number: 298-14-6
EINECS EC Number: 206-059-0
4. FIRST AID MEASURES
Always get medical attention after the first aid is over.
Emergency and First Aid Procedures:
Eyes - Flush with water for at least 15 minutes, raising and lowering eyelids occasionally. Get medical attention if irritation persists.
Skin - Thoroughly wash exposed area for at least 15 minutes. Remove contaminated clothing. Launder contaminated clothing before reuse. Get medical attention if irritation persists.
Ingestion - If Potassium bicarbonate is swallowed, if conscious, give plenty of water. Immediately call a physician. Never give anything by mouth to an unconscious person.
Inhalation - Remove to fresh air. Give oxygen if breathing is difficult; give artificial respiration if breathing has stopped. Get medical attention.
5. FIREFIGHTING PROCEDURES
Extinguisher Media: Use media suitable to extinguish surrounding fire.
Flammable Limits in Air % by Volume: N/A
Special Fire fighting Procedures: Firefighters should wear full protective equipment and NIOSH approved self-contained breathing apparatus.
6. SPILL OR LEAK PROCEDURES
Steps to be taken in Case Potassium Bicarbonate is Released or Spilled:
Ventilate area of leak or spill. Wear appropriate personal protective equipment.
Sweep up and containerize for reclamation or disposal. Vacuuming or wet sweeping may be used to avoid dust dispersal. Small amounts of residue may be flushed to sewer with plenty of water.
7. SPECIAL PRECAUTIONS
Keep Potassium bicarbonate in a well closed container stored under cold to warm conditions, 2 to 40 C, (36 to 104F). Protect against physical damage.
8. SPECIAL PROTECTION INFORMATION
Respiratory Protection (Specify Type): None needed under normal conditions of use with adequate ventilation. NIOSH approved equipment should be worn if PELs are exceeded.
Ventilation Local Exhaust: Yes
Eye Protection: Splash proof chemical safety goggles should be worn at all times.
Other Protective Clothing or Equipment: Lab apron, eye wash, and safety shower.
9. PHYSICAL DATA
Molecular Formula KHCO3
Molecular Weight 100.12
Appearance and Odor: Potassium bicarbonate is white crystalline powder, odorless
Melting Point: Decomposes before melting.
Boiling Point: N/A
Density: 2,17 g/cm3 at 20 °C
Vapor Pressure: N/A
Vapor Density (Air=1): N/A
Specific Gravity(H2O=1): 2.20
Percent Volatile by Volume: 0 (21C)
Evaporation Rate(H2O=1): N/A
Solubility in Water: Highly soluble. 362 g/l at 25 °C
10. REACTIVITY DATA
Stability: Potassium bicarbonate is stable
Conditions to Avoid: Heat, humidity
Incompatibility (Materials to Avoid): Strong oxidizing agents, Strong acids
Hazardous Decomposition Products: Carbon monoxide, carbon dioxide
Hazardous Polymerization: Will not occur
11. TOXICITY DATA
Toxicity Data:
LD50 Oral - rat - > 2.000 mg/kg
LD50 Dermal - rabbit - > 2.000 mg/kg
Conditions Aggravated by Overexposure: Skin and eye irritation
Carcinogenicity: No component of this product present at levels greater than or equal to 0.1% is identified as probable, possible or confirmed human carcinogen by IARC, NTP, ACGIH & OSHA.
12. ECOLOGICAL DATA
Toxicity to fish: LC50 - Oncorhynchus mykiss (rainbow trout) - 1.300 mg/l - 96 h
Toxicity to daphnia and other aquatic invertebrates: EC50 - Daphnia - 630 mg/l
Bioaccumulative potential: Does not bioaccumulate.
EPA Waste Numbers: None
13. DISPOSAL INFORMATION
Waste Disposal Methods: Dispose Potassium bicarbonate in accordance with all applicable Federal, State and Local regulations.
14. TRANSPORT INFORMATION
DOT: Not regulated.
IMDG: Not regulated.
IATA: Not regulated.
15. REGULATORY INFORMATION
SARA 311/312 Hazardous chemical: None present.
16. Other Information
Disclaimer:
******************************
Our company provides this MSDS information sheet contained herein in good faith but makes no representation as to its comprehensiveness or accuracy. This SDS sheet is intended only as a guide to the appropriate precautionary handling of the material by a properly trained person using this product. Individuals receiving the information must exercise their independent judgment in determining its appropriateness for a particular purpose.
******************************
Anmol Chemicals & Pharmaceuticals Pvt. Ltd. is an off-shoot of Anmol Chemicals Taloja. It is located in MIDC Taloja and it is manufacturing pharmaceutical grades of API, Excepients, Food grade and Reagent grade chemicals. Anmol Chemicals & Pharmaceuticals Pvt. Ltd. is a several decades old group of companies, engaged in manufacturing, supplying, distributing, wholesale supplies for actual users, retail or small pack supplies for research and development chemicals, fine and speciality chemicals, pharmaceutical excipients, mineral fortifiers in chemically pure, Analytical reagent grade, IP BP USP Ph Eur EP JP and other pharmaceutical grade monograph including FCC Food grade chemicals and Nutraceuticals, Mineral Fortifiers at best prices.
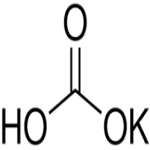
Potassium Bicarbonate Structure
CAS Number 298-14-6, Potassium Bicarbonate Manufacturer Exporter
ANMOL CHEMICALS & PHARMACEUTICALS Pvt. Ltd.
India, USA, Europe, UAE
TELEPHONE: +912223770100
Navi Mumbai, INDIA
e-mail: info(At the rate i.e. @)anmol.org
Copyright. 23-nov-24
We manufacture:

