CAS Number 134-03-2, Monosodium l-ascorbate or Sodium Ascorbate USP BP Ph Eur FCC Food Grade Manufacturers Exporters







CAS Number 134-03-2, Monosodium l-ascorbate or Sodium Ascorbate Manufacturer Exporter
For Properties Specifications of Monosodium l-ascorbate or Sodium Ascorbate Click Properties, Specifications of Monosodium l-ascorbate or Sodium Ascorbate Manufacturer.
For Uses of Monosodium l-ascorbate or Sodium Ascorbate Click Uses of Monosodium l-ascorbate or Sodium Ascorbate Manufacturer.
For For SDS MSDS Sheet of Monosodium l-ascorbate or Sodium Ascorbate Click SDS Safety Data Sheet MSDS Sheet of Monosodium l-ascorbate or Sodium Ascorbate Manufacturer.
The Properties and Specifications of Monosodium l-ascorbate or Sodium Ascorbate:
Specifications of Sodium Ascorbate USP Grade:
C6H7NaO6 --- 198.11
l-Ascorbic acid, monosodium salt;
Monosodium l-ascorbate CAS 134-03-2.
DEFINITION
Sodium Ascorbate contains NLT 99.0% and NMT 101.0% of sodium ascorbate (C6H7NaO6), calculated on the dried basis.
Optical Rotation, Specific Rotation: Acceptance criteria: +103° to +108°
pH: 7.0 to 8.0
Loss on Drying: Dry a sample in a suitable vacuum drying tube, using phosphorus pentoxide as the desiccant, at 60C for 4 h: it loses NMT 0.25% of its weight.
Specifications of Sodium Ascorbate BP Ph Eur Grade:
C6H7NaO6 --- 198.1 --- CAS 134-03-2
Action and use: Excipient.
DEFINITION
Sodium (2R)-2-[(1S)-1,2-dihydroxyethyl]-4-hydroxy-5-oxo-2,5-dihydrofuran-3-olate.
Content: 99.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance: White or yellowish, crystalline powder or crystals.
Solubility: Freely soluble in water, sparingly soluble in ethanol (96 per cent), practically insoluble in methylene chloride.
Specific optical rotation: + 103 to + 108 (dried substance), determined on freshly prepared solution S.
pH: 7.0 to 8.0 determined on freshly prepared solution S.
Sulfates: Maximum 150 ppm.
Copper: Maximum 5 ppm.
Iron: Maximum 2 ppm.
Nickel: Maximum 1 ppm.
Loss on drying: Maximum 0.25 per cent, determined on 1 g by drying in an oven at 105C.
Specified impurities: To pass the test.
Specifications of Sodium Ascorbate FCC Food Grade:
Vitamin C Sodium; Sodium L-Ascorbate
C6H7NaO6 Formula weight 198.11
INS: 301 CAS: 134-03-2
DESCRIPTION
Sodium Ascorbate occurs as a white to yellow, crystalline powder that does not darken on exposure to light. One gram is soluble in 2 mL of water. The pH of a 1:10 aqueous solution is about 7.5.
Function: Antioxidant; meat curing aid; nutrient.
REQUIREMENTS
Identification:
A. A 1:50 aqueous solution slowly reduces alkaline cupric tartrate at 25C, and does so more readily upon heating.
B. Add 4 drops of methylene blue to 2 mL of a 1:50 aqueous solution acidified with 0.5 mL of 0.1 N hydrochloric acid, and warm to 40C. The deep blue color disappears almost completely within 3 minutes.
C. Dissolve 15 mg of sample in 15 mL of a 1:20 trichloroacetic acid:water solution, add about 200 mg of activated charcoal, shake vigorously for 1 minute, and filter through a small fluted filter, returning the filtrate, if necessary, until clear. Add 1 drop of pyrrole to 5 mL of the filtrate, agitate gently until dissolved, and then heat in a water bath at 50C. A blue color appears.
D. A sample gives positive tests for Sodium.
Assay: Not less than 99.0% and not more than 101.0% of C6H7NaO6 after drying.
Lead: Not more than 2 mg/kg.
Loss on Drying: Not more than 0.25%.
Optical (Specific) Rotation [α]D25C: Between +103° and +108°.
The Uses of Monosodium l-ascorbate or Sodium Ascorbate:
Sodium ascorbate is a form of vitamin C that has sodium components that help lower its acidity levels. The sodium content helps vitamin C to be easily absorbed and stay longer in the body. It serves as an antioxidant that helps keep your cells from damage and keep them healthy. As a food additive, it has the E number E301 and is used as an antioxidant and an acidity regulator. It is approved for use as a food additive in the EU, USA, etc.
The MSDS-SDS Hazard Statement of Monosodium l-ascorbate or Sodium Ascorbate:
Sodium ascorbate or Monosodium l-ascorbate SDS, Safety Data Sheet
MSDS Sheet, Material Safety Data Sheet 22-Jan-23
Section 1: Chemical Product and Identification
Product Name & Other Names: Sodium ascorbate or Monosodium l-ascorbate or l-Ascorbic acid, monosodium salt or Vitamin C sodium salt.
CAS#: 134-03-2
EINECS EC-No.: 205-126-1
Chemical Formula: C6H7NaO6.
Molecular Weight: 198.10
Relevant uses and uses advised against (if any): Laboratory and Industrial Manufacturing.
Section 2: Hazards Identification
GHS, Globally Harmonized System Classification in accordance with 29 CFR 1910
Classification according to Regulation (EC) No 1272/2008
Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008.
This substance is not classified as dangerous according to Directive 67/548/EEC.
Labeling according to GHS & Regulation (EC) No 1272/2008
GHS Label Elements NONE |
Signal Word: None
Not considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200)
Precautionary statements:
P261: Avoid breathing dust/fume/gas/mist/vapors/spray.
P302+P352: IF ON SKIN: Wash with plenty of soap and water.
P303+P361+P353: IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse skin with water/shower.
P304+P340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.
P337+313: If eye irritation persists get medical advice/attention.
Section 3: Composition and Information on Ingredients
Product Name & Other Names: Sodium ascorbate or Monosodium l-ascorbate or l-Ascorbic acid, monosodium salt or Vitamin C sodium salt.
CAS#: 134-03-2
EINECS EC-No.: 205-126-1
Section 4: First Aid Measures
Always seek medical attention after first aid measures are provided.
Eye Contact: In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical attention.
Skin Contact: In case of contact, immediately flush skin with plenty of water. Cover the irritated skin with an emollient. Thoroughly clean shoes and clothes before reuse. Get medical attention.
Inhalation: If inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical attention.
Ingestion: Do NOT induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. If large quantities of this material are swallowed, call a physician immediately.
Section 5: Fire and Explosion Data
Flammability of the Product: It may be combustible at high temperature.
Products of Combustion: Some metallic oxides CO CO2 and fumes.
Fire Hazards in Presence of Various Substances: Slightly flammable in presence of heat. Non-flammable in presence of shocks.
Fire Fighting Media and Instructions:
Small Fire: Use DRY chemical powder.
Large Fire: Use water spray, fog, or foam. Avoid water jet.
Special Information: In the event of a fire, wear full protective clothing and NIOSH-approved self-contained breathing apparatus with full face piece operated in the pressure demand or other positive pressure mode. At high temperatures under fire conditions, it may produce toxic or irritating fumes. Fire-extinguishing work is done from the windward and the suitable fire-extinguishing method according to the surrounding situation is used.
Section 6: Accidental Release Measures
Personal precautions, protective equipment, and emergency procedures: Ventilate area of leak or spill. Avoid breathing dust/fumes/gas/mist/vapors/spray. Use individual protective equipment (waterproof boots, suitable protective clothing, safety glasses, etc.).Do not approach facing the wind.
Environmental precautions: Do not let the product enter drains, soil, or water sources.
Methods and materials used for containment Cleanup procedures and Storage: Contain spilled material. Cover with an inert, non-combustible absorbent material, (e.g. sand, earth, diatomaceous earth, vermiculite). Vacuum or sweep-up and remove to an approved disposal container.
Section 7: Handling and Storage
Precautions for safe handling: Apply according to good manufacturing and industrial hygiene practices. Ensure proper ventilation. Wash thoroughly after handling. Do not drink, eat, or smoke while handling. Avoid contact with skin, eyes, and clothing. Minimize dust generation. Avoid breathing dust/fumes/gas/mist/vapors/spray. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Use individual protective equipment (waterproof boots, suitable protective clothing, safety glasses, etc.).
Conditions for safe storage, including any incompatibilities: Store in cool, dry, and ventilated area away from heat sources and protected from sunlight in tightly closed original container. Keep air contact to a minimum. Store protected from heat, sparks and ignition sources and incompatible materials. Avoid contact with skin and eyes. Avoid inhalation of dust/mist/vapor. Do not store with incompatible materials like strong oxidizing agents.
Section 8: Exposure Controls/Personal Protection
Exposure Limits: Not established.
Engineering Controls: Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended exposure limits.
Ventilation System: A system of local and/or general exhaust is recommended to keep employee exposures as low as possible.
Personal Respirators (NIOSH Approved): For conditions of use where exposure to dust or mist is apparent and engineering controls are not feasible, a particulate respirator may be worn.
Skin Protection: Wear protective gloves and clean body-covering clothing.
Eye Protection: Use chemical safety goggles and/or full face shield where dusting or splashing of solutions is possible. Maintain eye wash fountain and quick-drench facilities in work area.
Other Control Measures: Maintain good housekeeping in work area. Handle in accordance with good industrial hygiene and safety practice.
Section 9: Physical and Chemical Properties
Physical state and appearance: White or yellowish, crystalline powder or crystals.
Odor: Odorless.
Odor threshold: Not available.
pH: 7.0 to 8.0 determined on freshly prepared solution.
Relative density: Not available.
Melting Point: 220C.
Initial boiling point and boiling range: 233C.
Flash point: Not available.
Auto-ignition temperature: Not available.
Decomposition temperature: Not available.
Upper/lower flammability or explosive limits: Not available.
Vapor pressure: Not available.
Vapor density: Not available.
Evaporation rate: Not available.
Flammability (solid, gas): Not available.
Partition coefficient: n-octanol/water: Not available.
Solubility: Freely soluble in water, sparingly soluble in ethanol (96 per cent), practically insoluble in methylene chloride.
Viscosity: Not available.
Section 10: Stability and Reactivity Data
Stability: It is stable.
Conditions of Instability: Excessive heat, dust generation
Incompatibility with various substances: Oxidizing Agents, heat and sunlight.
Special Remarks on Reactivity: Releases water of crystallization when heated and decomposes.
Hazardous decomposition products formed under fire conditions: Carbon oxides, Sodium oxide.
Polymerization: Will not occur.
Section 11: Toxicological Information
Toxicity to Animals: Not available.
Carcinogenicity: No component of this product present at levels greater than or equal to 0.1% is identified as probable or confirmed human carcinogen by IARC, ACGIH, OSHA and NTP.
Mutagenic Effects: Not available.
Environmental Toxicity: Not available.
Section 12: Ecological Information
Ecotoxicity: Not available.
Products of Biodegradation: Possibly hazardous short-term degradation products are not likely. However, long term degradation products may arise.
Toxicity of the Products of Biodegradation: The product itself and its products of degradation are not toxic.
Results of PBT and vPvB assessment: This substance/mixture contains no components considered to be either persistent, bioaccumulative and toxic (PBT), or very persistent and very bio accumulative (vPvB) at levels of 0.1% or higher.
Section 13: Disposal Considerations
Waste Disposal: Waste must be disposed of in accordance legal regulations.
Section 14: Transport Information
DOT USA, TDG Canada & ADR/RID Europe: Not controlled.
IMO/IMDG: Not controlled.
IATA: Not controlled.
Section 15: Other Regulatory Information
USA:
SARA 311/312 Hazard Categories: See section 2.
California Proposition 65: Not listed.
Disclaimer:
**************************
Our company provides this MSDS sheet in good faith but makes no representation as to its comprehensiveness or accuracy. This SDS sheet is intended only as a guide to the appropriate precautionary handling of the material by a properly trained person using this product. The above information has been compiled from various sources and has the possibility of discrepancy and being out-dated information. Individuals receiving the information must exercise their independent judgment and do further search in determining its appropriateness for a particular purpose. In no case shall our company be liable to loss or damages by the product user.
**************************
Anmol Chemicals & Pharmaceuticals Pvt. Ltd. is an off-shoot of Anmol Chemicals Taloja. It is located in MIDC Taloja and it is manufacturing pharmaceutical grades of API, Excepients, Food grade and Reagent grade chemicals. Anmol Chemicals & Pharmaceuticals Pvt. Ltd. is a several decades old group of companies, engaged in manufacturing, supplying, distributing, wholesale supplies for actual users, retail or small pack supplies for research and development chemicals, fine and speciality chemicals, pharmaceutical excipients, mineral fortifiers in chemically pure, Analytical reagent grade, IP BP USP Ph Eur EP JP and other pharmaceutical grade monograph including FCC Food grade chemicals and Nutraceuticals, Mineral Fortifiers at best prices.
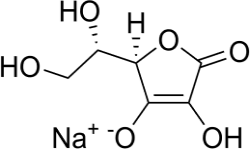
Monosodium l-ascorbate or Sodium Ascorbate Structure
CAS Number 134-03-2, Monosodium l-ascorbate or Sodium Ascorbate Manufacturer Exporter
ANMOL CHEMICALS & PHARMACEUTICALS Pvt. Ltd.
India, USA, Europe, UAE
TELEPHONE: +912223770100
Navi Mumbai, INDIA
e-mail: info(At the rate i.e. @)anmol.org
Copyright. 10-dec-24
We manufacture:
Glacial Acetic Acid Manufacturer
Aluminium Sodium Silicate or Sodium Aluminosilicate
Dihydroxyaluminum Aminoacetate

