CAS Number 7446-20-0, 13986-24-8, 7446-19-7 & 7733-02-0, Zinc Sulfate or Zinc Sulphate Anhydrous Monohydrate Hexahydrate Heptahydrate USP BP Ph Eur Analytical Reagent FCC Food Grade Manufacturers Exporters








CAS Number 7446-20-0, 13986-24-8, 7446-19-7 & 7733-02-0, Zinc Sulfate or Zinc Sulphate Anhydrous Monohydrate Hexahydrate Heptahydrate Manufacturer Exporter
For Properties Specifications of Zinc Sulfate or Zinc Sulphate Anhydrous Monohydrate Hexahydrate Heptahydrate Click Properties, Specifications of Zinc Sulfate or Zinc Sulphate Manufacturer.
For Uses of Zinc Sulfate or Zinc Sulphate Anhydrous Monohydrate Hexahydrate Heptahydrate Click Uses of Zinc Sulfate or Zinc Sulphate Anhydrous Monohydrate Hexahydrate Heptahydrate Manufacturer.
For For SDS MSDS Sheet of Zinc Sulfate or Zinc Sulphate Anhydrous Monohydrate Hexahydrate Heptahydrate Click SDS Safety Data Sheet MSDS Sheet of Zinc Sulfate or Zinc Sulphate Anhydrous Monohydrate Hexahydrate Heptahydrate Manufacturer.
The Properties and Specifications of Zinc Sulfate or Zinc Sulphate Anhydrous Monohydrate Hexahydrate Heptahydrate:
Zinc Sulfate USP Grade Specifications
ZnSO4-xH2O
Sulfuric acid, zinc salt (1:1), hydrate.
Zinc sulfate (1:1) monohydrate 179.46
Zinc sulfate (1:1) heptahydrate 287.56 CAS 7446-20-0
Anhydrous 161.46 CAS 7733-02-0
Zinc Sulfate contains one or seven molecules of water of hydration. The monohydrate contains not less than 89.0 percent and not more than 90.4 percent of ZnSO4, corresponding to not less than 99.0 percent and not more than 100.5 percent of ZnSO4-H2O, and the heptahydrate contains not less than 55.6 percent and not more than 61.0 percent of ZnSO4, corresponding to not less than 99.0 percent and not more than 108.7 percent of ZnSO4-7H2O.
Identification: A solution of it responds to the tests for Zinc and for Sulfate.
Acidity: A solution containing the equivalent of 28 mg of ZnSO4 per mL is not colored pink by methyl orange TS.
Arsenic: the limit is 14 ppm.
Lead: The lead limit is 0.002%.
Alkalies and alkaline earths: Dissolve the equivalent of 1.12 g of ZnSO4 in about 150 mL of water contained in a 200-mL volumetric flask. Precipitate the zinc completely by means of ammonium sulfide TS, and dilute with water to volume. Mix, and filter through a dry filter, rejecting the first portion of the filtrate. To 100 mL of the subsequent filtrate add a few drops of sulfuric acid, evaporate to dryness in a tarred dish, and ignite: the weight of the residue does not exceed 5 mg (0.9%).
Zinc Sulphate Heptahydrate BP Ph Eur Grade Specifications
ZnSO4,7H2O -- 287.5 -- CAS 7446-20-0
DEFINITION
Content: 99.0 per cent to 104.0 per cent Zinc Sulphate Heptahydrate ( Zinc Sulfate Heptahydrate ).
CHARACTERS
Appearance: White or almost white, crystalline powder or colourless, transparent crystals, efflorescent.
Solubility: Very soluble in water, practically insoluble in alcohol.
IDENTIFICATION
A. Solution S (see Tests) gives the reactions of sulphates.
B. Solution S gives the reaction of zinc.
C. It complies with the limits of the assay.
TESTS
Solution S: Dissolve 2.5 g in carbon dioxide-free water and dilute to 50 ml with the same solvent.
Appearance of solution: Solution S is clear and colourless.
pH: 4.4 to 5.6 for solution S.
Chlorides: Maximum 300 ppm.
Iron: Maximum 100 ppm.
Zinc Sulphate Hexahydrate BP Ph Eur Grade Specifications
ZnSO4-6H2O -- 269.5 -- CAS 13986-24-8
DEFINITION
Content: 99.0 per cent to 104.0 per cent Zinc Sulphate Hexahydrate ( Zinc Sulfate Hexahydrate ).
CHARACTERS
Appearance: White or almost white, crystalline powder or colourless transparent crystals, efflorescent.
Solubility: Very soluble in water, practically insoluble in alcohol.
IDENTIFICATION
A. Solution S (see Tests) gives the reactions of sulphates.
B. Solution S gives the reaction of zinc.
C. It complies with the limits of the assay.
TESTS
Solution S: Dissolve 2.5 g in carbon dioxide-free water and dilute to 50 ml with the same solvent.
Appearance of solution: Solution S is clear and colourless.
pH: 4.4 to 5.6 for solution S.
Chlorides: Maximum 300 ppm.
Iron: Maximum 100 ppm.
Zinc Sulphate Monohydrate BP Ph Eur Grade Specifications
ZnSO4-H2O -- 179.5 -- CAS 7446-19-7
DEFINITION
Content: 99.0 per cent to 101.0 per cent.
CHARACTERS
Appearance: White or almost white, crystalline powder, or colourless, transparent crystals.
Solubility: Very soluble in water, practically insoluble in ethanol (96 per cent).
IDENTIFICATION
A. Solution S (see Tests) gives the reactions of sulphates.
B. Solution S gives the reaction of zinc.
C. It complies with the limits of the assay.
TESTS
Solution S: Dissolve 2.5 g in carbon dioxide-free water R and dilute to 50 ml with the same solvent.
Appearance of solution: Solution S is clear and colourless.
pH: 4.4 to 5.6 for solution S.
Chlorides: Maximum 300 ppm.
Iron: Maximum 100 ppm.
Zinc Sulfate FCC Food Grade Specifications
ZnSO4-H2O Formula weight, monohydrate 179.45
ZnSO4-7H2O Formula weight, heptahydrate 287.54
CAS: monohydrate CAS 7446-19-7
CAS: heptahydrate CAS 7446-20-0
DESCRIPTION
Zinc Sulfate occurs as colorless, transparent prisms or small needles, or as a granular, crystalline powder. It contains one or seven molecules of water of hydration. The monohydrate loses water at temperatures above 238C; the heptahydrate effloresces in dry air at room temperature. Its solutions are acid to litmus. The monohydrate is soluble in water and practically insoluble in alcohol. One gram of the heptahydrate dissolves in about 0.6 mL of water and in about 2.5 mL of glycerin; it is insoluble in alcohol.
Function: Nutrient.
REQUIREMENTS
Identification: A 1:20 aqueous solution gives positive tests for Zinc and for Sulfate.
Assay: Monohydrate: Not less than 98.0% and not more than 100.5% of ZnSO4-H2O; Heptahydrate: Not less than 99.0% and not more than 108.7% of ZnSO4-7H2O.
Acidity: Passes test.
Alkalies and Alkaline Earths: Not more than 0.5%.
Cadmium: Not more than 2 mg/kg.
Lead: Not more than 4 mg/kg.
Mercury: Not more than 5 mg/kg.
Selenium: Not more than 0.003%.
Zinc Sulfate Analytical Reagent Grade Specifications
Zinc Sulfate Heptahydrate
ZnSO4-7H2O
Formula Weight 287.56
CAS Number 7446-20-0
Assay: 99.0-103.0% ZnSO4- 7H2O
pH of a 5% solution: 4.4-6.0 at 25C
MAXIMUM ALLOWABLE
Insoluble matter: 0.01%
Chloride (Cl): 5 ppm
Nitrate (NO3): 0.002%
Ammonium (NH4): 0.001%
Calcium (Ca): 0.005%
Iron (Fe): 0.001%
Lead (Pb): 0.003%
Magnesium (Mg): 0.005%
Manganese (Mn): 3 ppm
Potassium (K): 0.01% Sodium (Na): 0.05%.
The Uses of Zinc Sulfate or Zinc Sulphate Anhydrous Monohydrate Hexahydrate Heptahydrate:
Zinc sulfate is used medically as a dietary supplement. Specifically it is used to treat zinc deficiency and to prevent the condition in those at high risk. This includes use together with oral rehydration therapy for children who have diarrhea. Zinc is also used for acne, diabetes, anorexia, burns, and many other purposes.
The MSDS-SDS Hazard Statement of Zinc Sulfate or Zinc Sulphate Anhydrous Monohydrate Hexahydrate Heptahydrate:
Zinc Sulphate or Zinc Sulfate SDS Safety Data Sheet
MSDS, Material Safety Data Sheet 01-Feb-23
SECTION 1. PRODUCT IDENTIFICATION
Product Name & Other Names: Zinc Sulphate or Zinc Sulfate. It comes as heptahydrate, monohydrate and anhydrous.
CAS Number: 7446-19-7
EINECS EC Number: 231-793-3
Molecular Weight: 161.4 for anhydrous.
Chemical Formula: ZnSO4 for anhydrous.
Relevant uses and uses advised against (if any): Industrial Manufacturing.
SECTION 2. HAZARDS IDENTIFICATION
GHS, Globally Harmonized System Classification in accordance with 29 CFR 1910
Classification according to Regulation (EC) No 1272/2008
Acute toxicity oral (Category 1), H302
Skin corrosion/irritation (Category 2), H315
Serious eye damage (Category 1), H318
Specific target organ toxicity, single exposure; Respiratory tract irritation (Category 3), H335
Hazardous to the aquatic environment, long-term hazard (Category 1), H410
Labeling according to GHS USA & Regulation (EC) No 1272/2008
GHS Label Elements  Aquatic Toxicity |
GHS Label Elements |
Signal Words: Danger
Hazard statements:
H302: Harmful if swallowed
H315: Causes skin irritation.
H318: Causes serious eye damage.
H335: May cause respiratory irritation.
H410: Very toxic to aquatic life with long lasting effects.
Precautionary statements:
P261: Avoid breathing dust/ fume/ gas/ mist/ vapors/ spray.
P262: Do not get in eyes, on skin, or on clothing.
P264 Wash skin thoroughly after handling.
P270: Do not eat, drink or smoke when using this product.
P273: Avoid release to the environment.
P280: Wear protective gloves/protective clothing/eye protection/face protection.
P330: Rinse mouth.
P301+P312: IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell.
P302+P352: IF ON SKIN: Wash with soap and water.
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.
SECTION 3. COMPOSITION / INFORMATION ON INGREDIENTS
Product Name & Other Names: Zinc Sulphate or Zinc Sulfate. It comes as heptahydrate, monohydrate and anhydrous.
CAS Number: 7446-19-7
EINECS EC Number: 231-793-3
SECTION 4. FIRST AID MEASURES
Always seek medical attention after first aid measures are provided.
Eye Contact: Do not allow victim to rub eye (s). Let the eye (s) water naturally for a few minutes. If particle/dust does not dislodge, flush with lukewarm, gently flowing water for 5 minutes or until particle/dust is removed, while holding eyelid (s) open. If irritation persists, obtain medical attention. DO NOT attempt to manually remove anything stuck to the eye.
Skin Contact: Remove contaminated clothing, shoes, and leather goods (e.g. watchbands, belts). Quickly and gently blot or brush away excess chemical. Wash gently and thoroughly with lukewarm gently flowing water and non-abrasive soap for 5 minutes. If irritation persists, repeat flushing. Obtain medical advice. Completely decontaminate clothing, shoes and leather goods before reuse or else discard.
Inhalation: If symptoms are experienced, remove source of contamination or move victim to fresh air. Obtain medical advice.
Ingestion: Never give anything by mouth if victim is rapidly losing consciousness or is unconscious or convulsing. Have victim rinse mouth thoroughly with water. DO NOT INDUCE VOMITING. Have victim drink 2 – 8 oz. (60 – 240 ml) of water. Zinc sulfate is an emetic and may cause vomiting. If vomiting occurs naturally, have victim rinse mouth with water again. Obtain medical advice and bring a copy of this MSDS.
SECTION 5. FIRE FIGHTING MEASURES
Fire and Explosion Hazards: It does not burn or support combustion.
Extinguishing Media: Use any means of extinction appropriate for the surrounding fire conditions such as water spray, carbon dioxide, dry chemical, or foam.
Fire Fighting: Toxic fumes of sulfur dioxide may result from combustion. As with any fire, fire fighters should be fully trained and wear full protective clothing including an approved, self-contained breathing apparatus which supplies a positive air pressure within a full-face piece mask. Do not use water directly on material. Do not allow water run-off to enter sewers or watercourses.
Special Information: In the event of a fire, wear full protective clothing and NIOSH-approved self-contained breathing apparatus with full face piece operated in the pressure demand or other positive pressure mode. At high temperatures under fire conditions, it may produce toxic or irritating fumes. Fire-extinguishing work is done from the windward and the suitable fire-extinguishing method according to the surrounding situation is used.
SECTION 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment, and emergency procedures: Avoid breathing dust/fumes/gas/mist/vapors/spray. Use individual protective equipment (waterproof boots, suitable protective clothing, safety glasses, etc.). Do not approach facing the wind.
Environmental precautions: Do not let the product enter drains, soil, or water sources.
Methods and materials used for containment Cleanup procedures and Storage: Do not inhale dust, vapors, mist, or gas. Avoid dust formation. Contain spilled material. Cover with an inert, non-combustible absorbent material, (e.g. sand, earth, diatomaceous earth, vermiculite). Use a shovel to put the material into a convenient waste disposal container.
SECTION 7. HANDLING AND STORAGE
Precautions for safe handling: Apply according to good manufacturing and industrial hygiene practices. Ensure proper ventilation. In case of insufficient ventilation, wear suitable respiratory equipment. Wash thoroughly after handling. Do not drink, eat, or smoke while handling. Avoid contact with skin, eyes, and clothing. Minimize dust generation. Avoid breathing dust/fumes/gas/mist/vapors/spray. Keep container tightly closed. Avoid ingestion and inhalation. Use individual protective equipment (waterproof boots, suitable protective clothing, safety glasses, etc.). Prevent any contact with hot surfaces.
Conditions for safe storage, including any incompatibilities: Store in cool, dry, and ventilated area away from heat sources and protected from sunlight in tightly closed original container. Keep air contact to a minimum. Store protected from heat, sparks and ignition sources and incompatible materials. Avoid contact with skin and eyes. Avoid inhalation of dust/mist/vapor. Do not store with incompatible materials like strong oxidizing agents.
SECTION 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Exposure Limits: This product does not contain any hazardous materials with occupational exposure limits established by the region-specific regulatory bodies.
Ventilation System: A system of local and/or general exhaust is recommended to keep employee exposures as low as possible.
Personal Respirators (NIOSH Approved): For conditions of use where exposure to dust or mist is apparent and engineering controls are not feasible, a particulate respirator may be worn.
Skin Protection: Wear protective gloves and clean body-covering clothing.
Eye Protection: Use chemical safety goggles and/or full face shield where dusting or splashing of solutions is possible. Maintain eye wash fountain and quick-drench facilities in work area.
Other Control Measures: Maintain good housekeeping in work area. Handle in accordance with good industrial hygiene and safety practice.
SECTION 9. PHYSICAL AND CHEMICAL PROPERTIES
Appearance: It is colorless, odorless granules or powder.
Odor: None.
Odor threshold: Not available.
pH: 4 to 6 @ 10% solution
Relative density: around 3.28 for heptahydrate.
Freezing/Melting Point/Range: Loses water at 238C; Decomposes at 680C (1256F)
Initial boiling point and boiling range: Not available.
Flash point: Not available.
Auto-ignition temperature: Not available.
Decomposition temperature: Not available.
Upper/lower flammability or explosive limits: Not available.
Vapor pressure: Not available.
Vapor density: Not available.
Evaporation rate: Not available.
Flammability (solid, gas): Not available.
Partition coefficient: n-octanol/water: Not available.
Solubility in Water: Soluble in water. Aqueous solutions are acidic.
Viscosity: Not available.
SECTION 10. STABILITY AND REACTIVITY
Stability and Reactivity: Stable and not considered reactive under normal temperatures and pressures. Hazardous polymerization or runaway reactions will not occur.
Incompatibilities: Strong oxidizing agents. Avoid excessive heating.
Hazardous Decomposition Products: High temperature operations such as oxyacetylene cutting, electric arc welding or severe overheating will generate zinc oxide fume which, on inhalation in sufficient quantity, can produce metal fume fever. Under such conditions, sulfur dioxide will also be generated and can cause respiratory distress.
SECTION 11. TOXICOLOGICAL INFORMATION
Acute Toxicity (Oral LD50): > 574 mg/kg Rat
Acute Toxicity (Dermal LD50): > 2000 mg/kg Rat
General: In the form in which Zinc Sulphate is sold it is relatively non-toxic. The major route of exposure would be through the generation and inhalation of airborne dust and especially the generation of zinc oxide fume through thermal decomposition.
Acute:
Skin/Eye: Zinc Sulfate direct contact may cause local irritation of the eyes or skin but would not cause tissue damage. Eye contact with solutions (>1%) may cause the appearance of white flecks on the lens of the eye. Dust or fume from burning or welding operations may also cause local irritation.
Inhalation: Zinc Sulphate acute inhalation may result in irritation but is not expected to cause significant harmful effects. Symptoms may include discomfort, coughing, tingling sensation, sneezing and/or shortness of breath and wheezing. Extreme heating of Zinc Sulphate monohydrate will generate zinc oxide fume. If inhaled, this fume can result in the condition called metal fume fever. The symptoms of metal fume fever will occur within 3 to 10 hours of exposure, and include immediate dryness and irritation of the throat, tightness of the chest, and coughing which may later be followed by flu-like symptoms of fever, malaise, perspiration, frontal headache, muscle cramps, low back pain, occasionally blurred vision, nausea, and vomiting. The symptoms are temporary and generally disappear, without medical intervention, within 24 to 48 hours of onset. There are no recognized complications, after affects, or chronic affects that result from this condition.
Ingestion: Zinc Sulfate ingestion of large doses can cause anemia and stomach symptoms. It is very astringent, and when ingested in excessive quantities, can irritate the stomach, resulting in abdominal pain, nausea, diarrhea, and spontaneous vomiting.
Chronic: In general, zinc is a low toxicity metal. Zinc is an important trace element for humans and the body regulates the amount of zinc stored by decreasing absorption and increasing excretion when intake is increased. Industrial experience has not identified any significant chronic effects from zinc sulfate to date. The item is not listed as a carcinogen by the Occupational Safety and Health Administration (OSHA), the National Toxicology Program (NTP), the International Agency for Research on Cancer (IARC), the American Conference of Governmental Industrial Hygienists (ACGIH) or the European Union (EU).
Carcinogenicity: No component of this product present at levels greater than or equal to 0.1% is identified as possible or confirmed human carcinogen by IARC, ACGIH, OSHA and NTP.
Mutagenic Effects: Not available.
Developmental Toxicity: Not available.
Reproductive Effects: No information available.
SECTION 12. ECOLOGICAL INFORMATION
Eco toxicological data:
LC50 24 hours fish (rainbow trout) 1.24 mg/l
LC50 48 hours fish (rainbow trout) 2.4 - 5 mg/l
LC50 96 hours fish (rainbow trout) 0.24 - 0.83 mg/l
LC50 96 hours Daphnia 7.4 mg/l
Zinc Sulphate has high water solubility and its zinc content is directly bio available. The zinc may be toxic to aquatic organisms, especially fish, with water hardness, pH and dissolved organic carbon levels being regulating factors.
SECTION 13. DISPOSAL CONSIDERATIONS
Do not wash the material down drain. Put uncontaminated material back into the process if possible. Place contaminated material in suitable, labeled containers for disposal.
SECTION 14. TRANSPORT INFORMATION
DOT USA, TDG Canada & ADR/RID Europe:
UN Number: 3077
UN proper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S. (Zinc sulphate)
Transport hazard class(es): 9; Packaging group: III.
IATA/ICAO:
UN Number: 3077
UN proper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S. (Zinc sulphate)
Transport hazard class(es): 9; Packaging group: III.
IMDG/IMO:
UN Number: 3077
UN proper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S. (Zinc sulphate)
Transport hazard class(es): 9; Packaging group: III.
SECTION 15. REGULATORY INFORMATION
USA Regulations:
SARA 311/312: See section 2.
SECTION 16 - ADDITIONAL INFORMATION
DISCLAIMER: The information and recommendations set forth herein are presented in good faith and believed correct as of the date hereof. It is compiled from various sources and it is not necessarily all inclusive nor fully adequate in every circumstance. In addition, these suggestions should not be confused with nor followed in violation of applicable laws, regulations, rules or insurance requirements applicable. This MSDS sheet is intended only as a guide to the appropriate precautionary handling of the material by a properly trained person using this product. Individuals receiving the information must exercise their independent judgment in determining its appropriateness for a particular purpose.
Anmol Chemicals & Pharmaceuticals Pvt. Ltd. is an off-shoot of Anmol Chemicals Taloja. It is located in MIDC Taloja and it is manufacturing pharmaceutical grades of API, Excepients, Food grade and Reagent grade chemicals. Anmol Chemicals & Pharmaceuticals Pvt. Ltd. is a several decades old group of companies, engaged in manufacturing, supplying, distributing, wholesale supplies for actual users, retail or small pack supplies for research and development chemicals, fine and speciality chemicals, pharmaceutical excipients, mineral fortifiers in chemically pure, Analytical reagent grade, IP BP USP Ph Eur EP JP and other pharmaceutical grade monograph including FCC Food grade chemicals and Nutraceuticals, Mineral Fortifiers at best prices.
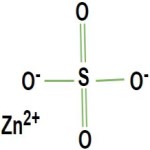
Zinc Sulfate or Zinc Sulphate Anhydrous Monohydrate Hexahydrate Heptahydrate Structure
CAS Number 7446-20-0, 13986-24-8, 7446-19-7 & 7733-02-0, Zinc Sulfate or Zinc Sulphate Anhydrous Monohydrate Hexahydrate Heptahydrate Manufacturer Exporter
ANMOL CHEMICALS & PHARMACEUTICALS Pvt. Ltd.
India, USA, Europe, UAE
TELEPHONE: +912223770100
Navi Mumbai, INDIA
e-mail: info(At the rate i.e. @)anmol.org
Copyright. 13-nov-24
We manufacture:
Calcium Gluceptate or Calcium Glucoheptonate
Copper Sulfate or Cupric Sulphate
Hydrated Sodium Glycerophosphate
TBHQ Tertiary Butylhydroquinone


