CAS Number 88-89-1, Picric Acid Reagent Grade Manufacturers Exporters







CAS Number 88-89-1, Picric Acid Manufacturer Exporter
For Properties Specifications of Picric Acid Click Properties, Specifications of Picric Acid Manufacturer.
For Uses of Picric Acid Click Uses of Picric Acid Manufacturer.
For For SDS MSDS Sheet of Picric Acid Click SDS Safety Data Sheet MSDS Sheet of Picric Acid Manufacturer.
The Properties and Specifications of Picric Acid:
Specification ---------------- General Grade ---------- LR Grade
Appearance ---------------- Lemon Yellow --------- Lemon Yellow
Assay: ------------------------ 98% ---------------------- 99,5%
Sulphate (SO4): ------------- 02% ---------------------- 0.05%
Melting range: --------------- 118-120°C ------------- 119-123°C
Insoluble and resinous matter: ------ ------------------- 0.2%
Analysis applied to the dry substance, Water added as agreed.
The Uses of Picric Acid:
Picric acid is used in the production of explosives, matches, and electric batteries. It is also used in etching copper and manufacturing colored glass, in the leather industry, and in the synthesis of dyes. Picric acid has antiseptic and astringent properties. For medical use it is incorporated in a surface anesthetic ointment or solution and in burn ointments.
The MSDS-SDS Hazard Statement of Picric Acid:
Picric acid SDS, Safety Data Sheet
MSDS Sheet, Material Safety Data Sheet 15-Jan-23
Section 1 - CHEMICAL PRODUCT AND COMPANY IDENTIFICATION
Product Name & Other Names: Picric acid
Synonyms: C6-H3-N3-O7, HOC6H2(NO2)3, "carbazotic acid", "C.I. 10305", "2-hydroxy-1, 3, 5-trinitrobenzene", "2-hydroxy-1, 3, 5-trinitrobenzene", melinite, "nitroxanthic acid", "phenol trinitrate", "picronitric acid", "bitter acid", "2, 4, 6-trinitrophenol", "2, 4, 6-trinitrophenol", "phenol-2, 4, 6-trinitro-", "phenol-2, 4, 6-trinitro-", "1, 3, 5-trinitrophenol", "1, 3, 5-trinitrophenol"
CAS: 88-89-1
EINECS EC Number: 201-865-9
Molecular Formula: C6H3N3O7
Molecular Weight: 229.10
Relevant uses and uses advised against (if any): For Industrial and Laboratory use only.
Section 2 - HAZARDS IDENTIFICATION
GHS, Globally Harmonized System Classification in accordance with 29 CFR 1910
Classification according to Regulation (EC) No 1272/2008
Explosives Division 1.1, H201
Flammable solids Category 1, H228
Self-reactive substances and mixtures Type B, H241
Acute oral toxicity Category 3 H301
Acute dermal toxicity Category 3, H311
Serious eye damage/eye irritation Category 2A, H319
Acute Inhalation Toxicity - Vapors Category 3, H331
Labeling according to GHS USA & Regulation (EC) No 1272/2008
GHS Label Elements  Toxic |
GHS Label Elements |
GHS Label Elements |
Signal Words: Danger
Hazard statements:
H201: Explosive; mass explosion hazard
H228: Flammable solid
H241: Heating may cause a fire or explosion.
H301: Toxic if swallowed.
H311: Toxic in contact with skin.
H319: Causes serious eye irritation.
H331: Toxic if inhaled.
Precautionary statements:
P210: Keep away from heat/sparks/open flames/hot surfaces – No smoking.
P220: Keep away from heat/sparks/open flames/hot surfaces – No smoking.
P230 :Keep wetted with water.
P234+235: Keep cool in original container only.
P240: Ground/bond container and receiving equipment.
P241 : Use explosion-proof electrical/ventilating/light/equipment.
P250: Do not subject to grinding/shock/friction.
P261: Avoid breathing dust/ fume/ gas/ mist/ vapors/ spray.
P262: Do not get in eyes, on skin, or on clothing.
P270: Do not eat, drink or smoke when using this product.
P271: Use only outdoors or in a well-ventilated area.
P280: Wear protective gloves/protective clothing/eye protection/face protection. P372: Explosion risk in case of fire.
P373: DO NOT fight fire when fire reaches explosives.
P301+310: IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician.
P302+352: IF ON SKIN: Wash with soap and water.
P304+340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.
P370+P378: In case of fire: Use water spray, fog or foam, dry chemical, CO2 for extinction.
P370+P380+375: In case of fire: Evacuate area. Fight fire remotely due to the risk of explosion.
P312: Call a POISON CENTER or doctor/physician if you feel unwell.
P314: Get Medical advice/attention if you feel unwell.
P337+P313: If eye irritation persists: Get medical advice/ attention.
P401: Store diluted with water.
P403+P233: Store in a well ventilated place. Keep container tightly closed.
P403+P235: Store in a well-ventilated place. Keep cool.
P420 : Store away from other materials.
POTENTIAL HEALTH EFFECTS AS PER LITERATURE:
Swallowed: Toxic effects may result from the accidental ingestion of the material; animal experiments indicate that ingestion of less than 40 gram may be fatal or may produce serious damage to the health of the individual.
Picric acid has an intense bitter taste. Several human poisonings have occurred after the ingestion of 1-2 grams picric acid. Symptoms include gastroenteritis, toxic hepatitis, inflammation of the kidney (nephritis), blood in the urine (haematuria) and other urinary symptoms.
Skin and conjunctiva become yellow due to acidosis and jaundice.
Death may follow renal lesions and anuria (both major kidney dysfunctions). Rarely, jaundice and coma with convulsions proceed death.
The substance and/or its metabolites may bind to hemoglobin inhibiting normal uptake of oxygen. This condition, known as "methemoglobinemia", is a form of oxygen starvation (anoxia).
Symptoms include cyanosis (a bluish discoloration skin and mucous membranes) and breathing difficulties. Symptoms may not be evident until several hours after exposure.
At about 15% concentration of blood methemoglobin there is observable cyanosis of the lips, nose, and earlobes. Symptoms may be absent although euphoria, flushed face and headache are commonly experienced. At 25-40%, cyanosis is marked but little disability occurs other than that produced on physical exertion. At 40-60%, symptoms include weakness, dizziness, lightheadedness, increasingly severe headache, ataxia, rapid shallow respiration, drowsiness, nausea, vomiting, confusion, lethargy, and stupor. Above 60% symptoms include dyspnea, respiratory depression, tachycardia or bradycardia, and convulsions. Levels exceeding 70% may be fatal.
Eye: Direct contact with the eye may produce transient discomfort characterized by tearing or conjunctival redness (as with windburn). Strange yellow vision is symptomatic of over-exposure, to picric acid, by this or other routes. Buffered solutions of picric acid produce lesions of significant severity in the range pH 1.5 to pH 9 with little significant difference.
Skin: Skin contact with the material may produce toxic effects; systemic effects may result following absorption. The material is not thought to be a skin irritant. Temporary discomfort, however, may result from prolonged dermal exposures. Good hygiene practice requires that exposure be kept to a minimum and that suitable gloves be used in an occupational setting. Skin absorption of picric acid may cause headache, vertigo with nausea, vomiting and rashes. Hair may be discolored yellow. Topical application may result in local or generalized allergic reaction. Entry into the bloodstream, though cuts, abrasions, or lesions, may produce systemic injury with harmful effects.
Inhaled: Inhalation of vapors or aerosols (mists, fumes), generated by the material during normal handling, may produce toxic effects. Inhalation of the material, especially for prolonged periods, may produce respiratory discomfort and occasionally, distress. Inhalation of high dust concentrations of picric acid may result in temporary unconsciousness followed by weakness, muscle pain, failure to produce urine (anuria) and later, excess urine production (polyuria).
Section 3 - COMPOSITION / INFORMATION ON INGREDIENTS
Product Name & Other Names: Picric acid
Synonyms: C6-H3-N3-O7, HOC6H2(NO2)3, "carbazotic acid", "C.I. 10305", "2-hydroxy-1, 3, 5-trinitrobenzene", "2-hydroxy-1, 3, 5-trinitrobenzene", melinite, "nitroxanthic acid", "phenol trinitrate", "picronitric acid", "bitter acid", "2, 4, 6-trinitrophenol", "2, 4, 6-trinitrophenol", "phenol-2, 4, 6-trinitro-", "phenol-2, 4, 6-trinitro-", "1, 3, 5-trinitrophenol", "1, 3, 5-trinitrophenol"
CAS: 88-89-1
EINECS EC Number: 201-865-9
Percent: 97-100% diluted with water. Containing not less than 30% water, for safety.
Section 4 - FIRST AID MEASURES
Always seek medical attention after first aid measures are provided.
SWALLOWED
If swallowed, refer for medical attention, where possible, without delay.
Where Medical attention is not immediately available or where the patient is more than 15 minutes from a hospital or unless instructed otherwise:
For advice, contact a Poisons Information Center or a doctor.
Urgent hospital treatment is likely to be needed.
If conscious, give water to drink.
Induce vomiting with fingers down the back of the throat, only if conscious. Lean patient forward or place on left side (head-down position, if possible) to maintain open airway and prevent aspiration.
NOTE: Wear a protective glove when inducing vomiting by mechanical means. If the services of a medical officer or medical doctor are readily available, the patient should be placed in his/her care and a copy of the MSDS should be provided. Further action will be the responsibility of the medical specialist. If medical attention is not available on the worksite or surroundings send the patient to a hospital together with a copy of the MSDS.
EYE
If this product comes in contact with the eyes, immediately hold eyelids apart and flush the eye continuously with running water. Ensure complete irrigation of the eye by keeping eyelids apart and away from eye and moving the eyelids by occasionally lifting the upper and lower lids. Continue flushing for at least 15 minutes. Transport to hospital or doctor without delay. Removal of contact lenses after an eye injury should only be undertaken by skilled personnel.
SKIN
If skin or hair contact occurs, quickly but gently, wipe material off skin with a dry, clean cloth. Immediately remove all contaminated clothing, including footwear. Wash skin and hair with running water. Transport to hospital, or doctor.
INHALED
If fumes or combustion products are inhaled remove from contaminated area. Lay patient down. Keep warm and rested. Prostheses such as false teeth, which may block airway, should be removed, where possible, prior to initiating first aid procedures. Apply artificial respiration if not breathing, preferably with a demand valve resuscitator, bag-valve mask device, or pocket mask as trained. Transport to hospital, or doctor, without delay.
NOTES TO PHYSICIAN
Symptoms of vasodilation and reflex tachycardia may present following organic nitrate overdose; most organic nitrates are extensively metabolized by hydrolysis to inorganic nitrites. Organic nitrates and nitrites are readily absorbed through the skin, lungs, mucosa and gastrointestinal tract.
The toxicity of nitrates and nitrites result from their vasodilating properties and their propensity to form methemoglobin.
Most produce a peak effect within 30 minutes.
Clinical signs of cyanosis appear before other symptoms because of the dark pigmentation of methemoglobin.
Initial attention should be directed towards improving oxygen delivery, with assisted ventilation, if necessary. Hyperbaric oxygen has not demonstrated conclusive benefits.
Institute cardiac monitoring, especially in patients with coronary artery or pulmonary disease.
Hypotension should respond to Trendelenburg's position and intravenous fluids; otherwise dopamine may be needed.
Naloxone, glucose and thiamine should be given if a multiple ingestion is suspected.
Decontaminate using Ipecac Syrup for alert patients or lavage for obtunded patients who present within 2-4 hours of ingestion.
Symptomatic patients with methemoglobin levels over 30% should receive methylene blue. (Cyanosis alone, is not an indication for treatment). The usual dose is 1-2 mg/kg of a 1% solution (10 mg/ml) IV over 5 minutes; repeat, using the same dose if symptoms of hypoxia fail to subside within 1 hour.
[Ellenhorn and Barceloux: Medical Toxicology] BIOLOGICAL EXPOSURE INDEX - BEI
These represent the determinants observed in specimens collected from a healthy worker who has been exposed at the Exposure Standard (ES or TLV):
Determinant Index Sampling Time Comments
1. Methemoglobin in blood 1.5% of hemoglobin During or end of shift B,NS,SQ
B: Background levels occur in specimens collected from subjects NOT exposed
NS: Non-specific determinant; also observed after exposure to other materials
SQ: Semi-quantitative determinant - Interpretation may be ambiguous; should be used as a screening test or confirmatory test.
Section 5 - FIRE FIGHTING MEASURES
Extinguishing media:
For SMALL FIRES: Dry chemical, CO2, water spray or foam.
For LARGE FIRES: Water-spray, fog, or foam.
Firefighting:
Alert Emergency Responders. Wear breathing apparatus plus protective gloves. Prevent, by any means available, spillage from entering drains or water course. Fight fire from a safe distance, with adequate cover. If safe, switch off electrical equipment until vapor fire hazard removed. Use water delivered as a fine spray to control fire and cool adjacent area. Avoid spraying water onto liquid pools.
DO NOT approach containers suspected to be hot. Cool fire exposed containers with water spray from a protected location. If safe to do so, remove containers from path of fire.
General Fire Hazards/Hazardous Combustible Products
WARNING: EXPLOSION HAZARD!
Combustible.
Detonation may occur from heavy impact or excessive heating.
Mixing with incompatible chemicals may cause expansion, decomposition or detonation.
Heat affected containers remain hazardous.
Explosives can supply own oxygen for combustion and smothering action of foam or dry chemical may be ineffective.
Combustion or decomposition produces oxides of nitrogen (NOx), carbon monoxide (CO) and carbon dioxide (CO2).
Fire Incompatibility
Avoid contamination with oxidizing agents i.e. nitrates, oxidizing acids, chlorine bleaches, pool chlorine etc. as ignition may result.
Personal Protection
Glasses:
Chemical goggles.
Gloves: 1.NEOPRENE 2.NITRILE 3.PVC
Respirator: Type AB-P Filter of sufficient capacity.
Section 6 - ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment, environmental precautions and emergency procedures: Avoid breathing dust/fumes/gas/mist/vapors/spray. Use individual protective equipment (waterproof boots, suitable protective clothing, safety glasses, etc). Restrict unprotected personnel from the area. Prevent any contact with hot surfaces. Do not approach facing the wind. Do not touch the spilled material. Keep away from drains, surface and groundwater and soil.
Methods and materials used for containment Cleanup procedures and Storage: Contain spilled material. Cover with an inert, non-combustible absorbent material, (e.g. sand, earth, diatomaceous earth, vermiculite), sweep up, and remove to an approved disposal container.
Minor Spills: Remove all ignition sources. DO NOT touch or walk through spilled material. Clean up all spills immediately. Avoid contact with skin and eyes. Prevent dust cloud. With clean shovel (preferably non-sparking) place material into clean, dry container and cover loosely. Move containers from spill area. Control personal contact by using protective equipment.
Major Spills: Clear area of personnel and move upwind. Alert Emergency Responders and tell them location and nature of hazard. DO NOT touch or walk through spilled material. Control personal contact by using protective equipment. Prevent, by any means available, spillage from entering drains or water course. No smoking, naked lights, or ignition sources. Increase ventilation. Stop leak if safe to do so. Contain or cover with sand, earth, or vermiculite. Use only spark-free shovels and explosion proof equipment. Collect recoverable product into labeled containers for recycling. Collect solid residues and seal in labeled drums for disposal. Wash area with water and dike for later disposal; prevent runoff into drains. After cleaning up operations, decontaminate and launder all protective clothing and equipment before storing and re-using. If contamination of drains or waterways occurs, advise emergency services.
Acute Exposure Guideline Levels (AEGL) (in ppm)
AEGL 1: The airborne concentration of a substance above which it is predicted that the general population, including susceptible individuals, could experience notable discomfort, irritation, or certain asymptomatic nonsensory effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
AEGL 2: The airborne concentration of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape.
AEGL 3: The airborne concentration of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
Section 7 - HANDLING AND STORAGE
Procedure For Safe Handling
Outside or detached storage is preferred
Containers should be bonded and grounded for transfers to avoid sparks
Use non-sparking tools and equipment including explosion proof ventilation
Store in glass NOT metals containers and wet screw tops before sealing
Do not store on concrete floors or wooden pallets
Enclose all processes and employ automatic mechanical handling techniques and wet methods where possible
If handling picric acid contained in a jar, gently tilt bottle to see if crystals roll over each other - if they do the acid is dry and capable of explosion - contact personnel trained in the handling of explosives immediately
Dry crystals may be present in the threads of screw top containers and present a detonation hazard when opening the container. Becomes Explosive when dry.
Containers of this material remain hazardous when empty since they retain product residues - observe all warnings for the product
Avoid all personal contact, including inhalation.
Wear protective clothing when risk of overexposure occurs.
Use in a well-ventilated area.
Prevent concentration in hollows and sumps.
DO NOT enter confined spaces until atmosphere has been checked.
DO NOT allow material to contact humans, exposed food or food utensils.
Avoid smoking, naked lights or ignition sources.
When handling, DO NOT eat, drink or smoke.
Avoid contact with incompatible materials.
Avoid reaction with oxidizing agents, bases and strong reducing agents.
Keep containers securely sealed when not in used.
Avoid physical damage to containers.
Always wash hands with soap and water after handling.
Working clothes should be laundered separately. Launder contaminated clothing before re-use.
Use good occupational work practice.
Recommended Storage Methods
DO NOT use unlined steel containers
Store in a dark glass or other suitable light resistant container.
For low viscosity materials and solids: Drums and jerricans must be of the non-removable head type. Where a can is to be used as an inner package, the can must have a screwed enclosure. For materials with a viscosity of at least 2680 cSt. (23 deg. C):
Removable head packaging and cans with friction closures may be used.
Where combination packages are used, there must be sufficient inert absorbent material to absorb completely any leakage that may occur, unless the outer packaging is a close fitting molded plastic box and the substances are not incompatible with the plastic. All combination packages for Packing group I and II must contain cushioning material.
Storage Requirements
For Minor Quantities: Store in an indoor fireproof cabinet or in a room of noncombustible construction Provide adequate portable fire-extinguishers in or near the storage area.
For Package Storage: Store in original containers in approved flame-proof area. No smoking, naked lights, heat, or ignition sources. DO NOT store in pits, depressions, basements, or areas where vapors may be trapped. Keep containers securely sealed. Store away from incompatible materials in a cool, dry well-ventilated area. Protect containers against physical damage and check regularly for leaks.
Protect containers from exposure to weather and from direct sunlight unless: (a) the packages are of metal or plastic construction; (b) the packages are securely closed are not opened for any purpose while in the area where they are stored and (c) adequate precautions are taken to ensure that rain water, which might become contaminated by the dangerous goods, is collected and disposed of safely. Ensure proper stock-control measures are maintained to prevent prolonged storage of dangerous goods.
Section 8 - EXPOSURE CONTROLS / PERSONAL PROTECTION
Exposure Limits| Chemical Name | ACGIH --------- and NIOSH ----------------------------- and OSHA - Final PELs |
Picric acid |
0.1 mg/m3 TWA and 0.1 mg/m3 TWA 75 mg/m3 IDLH and 0.1 mg/m3 TWA |
The TLV-TWA is thought to be protective against the development of systemic toxicity but may not, however, prevent the development of dermatitis or sensitization in some workers exposed at the 8-hour TWA.
Personal Protection: Consult your EHS staff for recommendations
Eye: Safety glasses with side shields. Chemical goggles. Contact lenses pose a special hazard; soft lenses may absorb irritants and all lenses concentrate them. DO NOT wear contact lenses.
Hands/Feet: NOTE: The material may produce skin sensitization in predisposed individuals. Care must be taken, when removing gloves and other protective equipment, to avoid all possible skin contact. Neoprene gloves; Wear physical protective gloves, e.g. leather.; Wear safety footwear.
Other:
Overalls.
Eyewash unit.
Barrier cream.
Skin cleansing cream.
Some plastic personal protective equipment (PPE) (e.g. gloves, aprons, overshoes) are not recommended as they may produce static electricity.
For large scale or continuous use wear tight-weave non-static clothing (no metallic fasteners, cuffs or pockets), non sparking safety footwear.
Respirators may be necessary when engineering and administrative controls do not adequately prevent exposures.
The decision to use respiratory protection should be based on professional judgment that takes into account toxicity information, exposure measurement data, and frequency and likelihood of the worker's exposure - ensure users are not subject to high thermal loads which may result in heat stress or distress due to personal protective equipment (powered, positive flow, full face apparatus may be an option).
Use approved positive flow mask if significant quantities of dust becomes airborne.
Try to avoid creating dust conditions.
Use appropriate NIOSH-certified respirator based on informed professional judgment. In conditions where no reasonable estimate of exposure can be made, assume the exposure is in a concentration IDLH and use NIOSH-certified full face pressure demand SCBA with a minimum service life of 30 minutes, or a combination full face piece pressure demand SAR with auxiliary self-contained air supply. Respirators provided only for escape from IDLH atmospheres shall be NIOSH-certified for escape from the atmosphere in which they will be used.
Engineering Controls
Local exhaust ventilation usually required. If risk of overexposure exists, wear an approved respirator. Correct fit is essential to obtain adequate protection an approved self-contained breathing apparatus (SCBA) may be required in some situations. Provide adequate ventilation in warehouse or closed storage area.
Air contaminants generated in the workplace possess varying "escape" velocities which, in turn, determine the "capture velocities" of fresh circulating air required to effectively remove the contaminant.
For large scale or continuous use: Spark-free, earthed ventilation system, venting directly to the outside and separate from usual ventilation systems Provide dust collectors with explosion vents.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Appearance: Yellow crystals with a very bitter taste supplied in water as paste.
Odor: Not available.
Odor threshold: Not available.
pH: Not available.
Relative density: 1.76for dry material.
Melting Range (°F): 251.6F
Boiling Range (°F): explodes >572F
Flash point: 302F
Auto-ignition temperature: 572F (explodes).
Decomposition temperature: Not available.
Upper/lower flammability or explosive limits: Not available.
Vapor Pressure (mmHG): < 0.26 @ 195C
Relative Vapor Density (air=1): 7.91 (anh.)
Evaporation rate: Not available.
Flammability (solid, gas): Not available.
Partition coefficient: n-octanol/water: Not available.
Solubility(ies): Soluble in alcohol, ether, acetone, benzene, acetic acid. Partly miscible with water.
Viscosity: Not available.
Explosive when dry.
Section 10 - CHEMICAL STABILITY
Stable under recommended storage conditions.
Conditions Contributing To Instability:
Presence of shock and friction
Presence of heat source and ignition source
Presence of incompatible materials.
Product is considered stable.
Hazardous polymerization will not occur.
Storage Incompatibility:
Picric acid when dry is a highly unstable and heat, friction-, and impact- sensitive explosive (explodes at approximately 300 C) explosive sensitivity increases when trace metals are present reacts with nitric acid, alkalis, heavy metals, copper, lead, zinc, transition metals, and other metals and their salts, to form other salts known as picrates; these are initiators which are much more highly sensitive to heat, impact, or shock than the parent compound – shock sensitive salts include ammonium salts and calcium salts (the calcium salt may be produced when picric acid comes in contact with plaster or concrete - do NOT store on uncoated concrete) mixtures with perchlorates form extremely powerful, high velocity explosives reacts violently with strong oxidizers is incompatible with ammonia may accumulate static electric charge, when dry, producing explosion is a strong oxidizer in aqueous solution and a strong acid reacts violently with alkalis, reducing agents, combustible materials, organic materials and other easily oxidized materials, aluminum powders, and other metal powders attacks many materials, forming flammable hydrogen gas attacks natural rubber, polyvinyl alcohol, PVC, Polynitro derivatives of mono- and poly- cyclic systems are often explosives liable to detonate on grinding or impact. The presence of two or more nitro groups (each with 2 oxygen atoms) on an aromatic nucleus often increase the reactivity of other substituents and the tendency towards explosive instability as oxygen balance is approached. Aromatic nitro compounds range from slight to strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides, and nitrides, they may begin a vigorous reaction that culminates in a detonation. The explosive tendencies of aromatic nitro compounds are increased by the presence of multiple nitro groups.
In view of the reports of previous violent or explosive reactions, heating of polynitroaryl (particularly di- and tri-nitroaryl) compounds with alkalies, ammonia, or O-ethylsulfuric acid salts, in autoclaves should be avoided.
Nitroaromatic and in particular polynitroaromatic compounds may present a severe explosion risk if subjected to shock or heated rapidly and uncontrollably as in fire situations. In addition, when such compounds are heated more moderately with caustic alkalies, even when water or organic solvents are present, there is also a risk of violent decomposition or explosion. Several industrial accidents, which probably were due to such interactions, have occurred; this potential hazard often remains unacknowledged.
A range of exothermic decomposition energies for nitro compounds is given as 220-410 kJ/mol. The relationship between energy of decomposition and processing hazards has been the subject of discussion; it is suggested that values of energy released per unit of mass, rather than on a molar basis (J/g) be used in the assessment. For example, in "open vessel processes" (with man-hole size openings, in an industrial setting), substances with exothermic decomposition energies below 500 J/g are unlikely to present a danger, whilst those in "closed vessel processes" (opening is a safety valve or bursting disk) present some danger where the decomposition energy exceeds 150 J/g.
Avoid reaction with oxidizing agents, bases and strong reducing agents.
Section 11 - TOXICOLOGICAL INFORMATION
Toxicity Data:
Oral (rat) LD50: 200 mg/kg Nil Reported
Intraperitoneal (mouse) LD50: 56.3 mg/kg
Oral (rabbit) LDLo: 120 mg/kg
Oral (cat) LDLo: 250 mg/kg
Mutagenic Effects: Mutagenic for bacteria and/or yeast. Causes damage to the following organs: mucous membranes.
Section 12 - ECOLOGICAL INFORMATION
Fish LC50 (96hr.) (mg/l): 30
Daphnia magna EC50 (48hr.) (mg/l): 88
log Pow (Verschueren 1983): 2.03
Harmful to aquatic organisms.
The nitrates are of environmental concern because of their high-water solubility and consequent leaching, diffusion, and environmental mobility in soil and water. Nitrate can contaminate groundwater to unacceptable levels. Nitrite is formed from nitrate or ammonium ion by micro-organisms in soil, water, sewage, and the alimentary tract. The concern with nitrate in the environment is related to its conversion to nitrite. Methemoglobinemia is caused following exposure to high levels of nitrite and produces difficulties in oxygen transport in the blood. Thousands of cases involving poisoning of infants, particularly in rural areas, have been reported because of drinking nitrate rich well-water. Other concerns deriving from exposure to environmental nitrates relate to the production of nitrosamines following the reaction of food nitrites and secondary amines. Other nitroso-compounds may result following reaction with nitrites and amides, ureas, carbamates and other nitrogenous compounds. Nitrosamines produce liver damage, hemorrhagic lung lesions, convulsions and coma in rats, and teratogenic effects in experimental animals.
The N-nitroso class of compounds include potent carcinogens and mutagens: induction of tumors by single doses of N-nitroso compounds testify to this.
DO NOT discharge into sewer or waterways.
Results of PBT and vPvB assessment: This substance/mixture contains no components considered to be either persistent, bioaccumulative and toxic (PBT), or very persistent and very bioaccumulative (vPvB) at levels of 0.1% or higher.
Section 13 - DISPOSAL CONSIDERATIONS
US EPA Waste Number & Descriptions
A. General Product Information
Ignitability characteristic: use EPA hazardous waste number D001 (waste code I)
Disposal Instructions:
All waste must be handled in accordance with local, state and federal regulations.
# Puncture containers to prevent re-use and bury at an authorized landfill.
Explosives which are surplus, deteriorated or considered unsafe for transport, storage or use shall be destroyed and the statutory authorities shall be notified.
Explosives must not be thrown away, buried, discarded or placed with garbage.
This material may be disposed of by burning or detonation but the operation must be performed under the control of a person competent in the destruction of explosives.
Disposal by detonation:
The explosives to be destroyed must be placed in direct contact with fresh priming charge in a hole which is at least 0.6 meter deep and then adequately stemmed.
No detonators shall be inserted into defective explosives.
Personnel must be evacuated to a safe distance prior to initiation/firing of the charge. Disposal by burning:
Make a sawdust bed or trail adequate for the quantity of explosives to be burned, approximately 400mm wide and 40 mm deep, upon which the explosive will be laid.
If sawdust is not available, newspaper may be used.
Normal precautions shall be taken to avoid the spread of fire.
Individual trails should not be closer together than 600 mm and should contain not more than 12 Kg of explosive.
Trails should be side by side, NOT in-line, and not more than four should be set up at one time.
Remove any explosive that is not to be burnt to a distance of at least 300 meter.
Sufficient diesel oil (never petrol or other highly inflammable liquid) should be used to thoroughly wet the sawdust (or paper) at least 4L per trail is recommended.
Light the trail from a long, rolled paper wick which should be placed downwind and in contact with the end 1m of trail that is not covered with explosive. The wind should blow so that the flame from the wick (and later from the burning explosive) will blow away from the unburned explosive as detonation is more likely to occur if the explosive is preheated by the flame.
If plastic igniter cord (slow) is available, its use for lighting is recommended instead of paper. One end should be coiled into the sawdust or under the paper and the other end lit from a minimum distance of 7m from the trail.
Retire at least 300m or to a safe place.
DO NOT return to the site for at least 30 minutes after the burning has apparently finished.
If the fire goes out do not approach for at least 15 minutes after all trace of fire has gone.
DO NOT add more diesel oil unless certain that the flame is completely extinguished.
Section 14 - TRANSPORTATION INFORMATION
DOT USA, TDG Canada & ADR/RID Europe:
Symbols: None
Hazard class or Division: 4.1
Identification Numbers: UN1344 PG: I
Label Codes: 4.1 Special provisions: 23, A8, A19, N41
Packaging: Exceptions: None Packaging: Non-bulk: 211
Hazard Alert Code Key: EXTREME HIGH MODERATE LOW
Packaging: Exceptions: None
Quantity limitations: Passenger aircraft/rail: 1 kg
Quantity Limitations: Cargo aircraft only: 15 kg Vessel stowage: Location: E
Vessel stowage: Other: 28, 36
Hazardous materials descriptions and proper shipping names: Trinitrophenol, wetted or Picric acid, wetted, with not less than 30 percent water by mass
Air Transport IATA:
ICAO/IATA Class: 4.1 ICAO/IATA Subrisk: !$
UN/ID Number: 1344 Packing Group: I
Special provisions: A40
Shipping Name: PICRIC ACID, WETTED
Maritime Transport IMDG (also IMO:
IMDG Class: 4.1 IMDG Subrisk: None
UN Number: 1344 Packing Group: I
EMS Number: F-B,S-J Special provisions: 28
Limited Quantities: None
Shipping Name: TRINITROPHENOL, WETTED with not less than 30% water, by mass.
Section 15 - REGULATORY INFORMATION
USA FEDERAL
California Prop 65: None of the chemicals in this product are listed.
Section 16 - Additional Information
Disclaimer:
*****************************
Our company provides this MSDS sheet in good faith but makes no representation as to its comprehensiveness or accuracy. This SDS sheet is intended only as a guide to the appropriate precautionary handling of the material by a properly trained person using this product. The above information has been compiled from various sources and has the possibility of discrepancy and being out-dated information. Individuals receiving the information must exercise their independent judgment and do further search in determining its appropriateness for a particular purpose. In no case shall our company be liable to loss or damages by the product user.
*****************************
Anmol Chemicals & Pharmaceuticals Pvt. Ltd. is an off-shoot of Anmol Chemicals Taloja. It is located in MIDC Taloja and it is manufacturing pharmaceutical grades of API, Excepients, Food grade and Reagent grade chemicals. Anmol Chemicals & Pharmaceuticals Pvt. Ltd. is a several decades old group of companies, engaged in manufacturing, supplying, distributing, wholesale supplies for actual users, retail or small pack supplies for research and development chemicals, fine and speciality chemicals, pharmaceutical excipients, mineral fortifiers in chemically pure, Analytical reagent grade, IP BP USP Ph Eur EP JP and other pharmaceutical grade monograph including FCC Food grade chemicals and Nutraceuticals, Mineral Fortifiers at best prices.
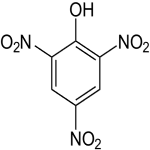
Picric Acid Structure
CAS Number 88-89-1, Picric Acid Manufacturer Exporter
ANMOL CHEMICALS & PHARMACEUTICALS Pvt. Ltd.
India, USA, Europe, UAE
TELEPHONE: +912223770100
Navi Mumbai, INDIA
e-mail: info(At the rate i.e. @)anmol.org
Copyright. 11-dec-24
We manufacture:
Glacial Acetic Acid Manufacturer
Fumed Silica or Colloidal Silicon Dioxide



