CAS Number 7758-87-4 or 12167-74-7 or 1306-06-5 or 62974-97-4, Tricalcium Phosphate or Calcium Phosphate Tribasic or Calcium Hydroxide Phosphate USP BP Ph Eur FCC Food Grade Manufacturers Exporters







CAS Number 7758-87-4 or 12167-74-7 or 1306-06-5 or 62974-97-4, Tricalcium Phosphate or Calcium Phosphate Tribasic or Calcium Hydroxide Phosphate Manufacturer Exporter
For Properties Specifications of Tricalcium Phosphate or Calcium Phosphate Tribasic or Calcium Hydroxide Phosphate Click Properties, Specifications of Tricalcium Phosphate or Calcium Phosphate Tribasic or Calcium Hydroxide Phosphate Manufacturer.
For Uses of Tricalcium Phosphate or Calcium Phosphate Tribasic or Calcium Hydroxide Phosphate Click Uses of Tricalcium Phosphate or Calcium Phosphate Tribasic or Calcium Hydroxide Phosphate Manufacturer.
For For SDS MSDS Sheet of Tricalcium Phosphate or Calcium Phosphate Tribasic or Calcium Hydroxide Phosphate Click SDS Safety Data Sheet MSDS Sheet of Tricalcium Phosphate or Calcium Phosphate Tribasic or Calcium Hydroxide Phosphate Manufacturer.
The Properties and Specifications of Tricalcium Phosphate or Calcium Phosphate Tribasic or Calcium Hydroxide Phosphate:
Tribasic Calcium Phosphate USP
Tribasic Calcium Phosphate
Ca5(OH)(PO4)3 502.31
Calcium hydroxide phosphate (Ca5(OH)(PO4)3).
Calcium hydroxide phosphate (Ca5(OH)(PO4)3) CAS 12167-74-7
Tribasic Calcium Phosphate consists of a variable mixture of calcium phosphates having the approximate composition 10CaO·3P2O5·H2O. It contains not less than 34.0 percent and not more than 40.0 percent of calcium (Ca).
Identification:
A: To a warm solution in a slight excess of nitric acid add ammonium molybdate TS: a yellow precipitate is formed.
B: It responds to the flame test for Calcium.
Loss on ignition: Ignite it at 800 for 30 minutes: it loses not more than 8.0% of its weight.
Water-soluble substances: Digest 2 g with 100 mL of water on a steam bath for 30 minutes, cool, add sufficient water to restore the original volume, stir well, and filter. Evaporate 50 mL of the filtrate in a tarred porcelain dish on a steam bath to dryness, and dry the residue at 120 to constant weight: the weight of the residue does not exceed 5 mg (0.5%).
Acid-insoluble substances: If an insoluble residue remains in the test for Carbonate, boil the solution, filter, wash the residue well with hot water until the last washing is free from chloride, and ignite the residue to constant weight: the weight of the residue does not exceed 4 mg (0.2%).
Carbonate: Mix 2 g with 20 mL of water, and add 3 N hydrochloric acid, drop wise, to effect solution: no effervescence is produced.
Chloride: Dissolve 500 mg in 25 mL of 2 N nitric acid, and add 1 mL of silver nitrate TS: the turbidity does not exceed that produced by 1.0 mL of 0.020 N hydrochloric acid (0.14%).
Sulfate: Dissolve 500 mg in the smallest possible amount of 3 N hydrochloric acid, dilute with water to 100 mL, filter, if necessary, and to 25 mL of the filtrate add 1 mL of barium chloride TS: the turbidity does not exceed that produced by 1.0 mL of 0.020 N sulfuric acid (0.8%).
Arsenic : The limit is 3 ppm.
Barium: Mix 500 mg with 10 mL of water, heat, add hydrochloric acid drop-wise until solution is effected, and then add 2 drops of the acid in excess. Filter, and add to the filtrate 1 mL of potassium sulfate TS: no turbidity appears within 15 minutes.
Dibasic salt and calcium oxide: Weigh accurately about 1.5 g, and dissolve by warming with 25.0 mL of 1 N hydrochloric acid VS. Cool, and slowly titrate the excess of 1 N hydrochloric acid, while agitating constantly, with 0.1 N sodium hydroxide VS to a pH of 4.0, determined potentiometrically. Not less than 13.0 mL and not more than 14.3 mL of 1 N hydrochloric acid is consumed for each g of salt, calculated on the ignited basis.
Limit of fluoride: [NOTE—Prepare and store all solutions in plastic containers.]: The limit is 0.0075%.
Limit of nitrate: Mix 200 mg with 5 mL of water, and add just sufficient hydrochloric acid to effect solution. Dilute with water to 10 mL, add 0.20 mL of indigo carmine TS, then add, with stirring, 10 mL of sulfuric acid: the blue color persists for not less than 5 minutes.
Tribasic Calcium Phosphate BP Ph Eur
Calcium Phosphate BP
CAS 7758-23-8
DEFINITION
Calcium phosphate consists of a mixture of calcium phosphates. It contains not less than 35.0 per cent and not more than the equivalent of 40.0 per cent of Ca (Ar 40.08).
CHARACTERS
A white or almost white powder, practically insoluble in water. It dissolves in dilute hydrochloric acid and in dilute nitric acid.
IDENTIFICATION
A. Dissolve 0.1 g in 5 ml of a 25 per cent V/V solution of nitric acid R. The solution gives reaction (b) of phosphates (2.3.1).
B. It gives reaction (b) of calcium (2.3.1). Filter before adding potassium ferrocyanide solution R.
C. It complies with the limits of the assay.
TESTS
Solution S: Dissolve 2.50 g in 20 ml of dilute hydrochloric acid. If the solution is not clear, filter it. Add dilute ammonia drop-wise until a precipitate is formed. Dissolve the precipitate by adding dilute hydrochloric acid and dilute to 50 ml with distilled water.
Chlorides: Dissolve 0.22 g in a mixture of 1 ml of nitric acid and 10 ml of water and dilute to 100 ml with water. 15 ml of the solution complies with the limit test for chlorides (0.15 per cent).
Fluorides: Not more than 75 ppm of F, determined potentiometrically (2.2.36, Method I) using a fluoride-selective indicator electrode and a silver-silver chloride reference electrode.
Sulphates: Dilute 1 ml of solution S to 25 ml with distilled water 15 ml of the solution complies with the limit test for sulphates (0.5 per cent).
Arsenic: 5 ml of solution S complies with limit test A for arsenic (4 ppm).
Iron: Dilute 0.5 ml of solution S to 10 ml with water R. The solution complies with the limit test for iron (400 ppm).
Heavy metals: Dilute 13 ml of solution S to 20 ml with water. 12 ml of the solution complies with limit test A for heavy metals (30 ppm).
Acid-insoluble matter: Dissolve 5.0 g in a mixture of 10 ml of hydrochloric acid and 30 ml of water. Filter, wash the residue with water and dry to constant mass at 100°C to 105 °C. The residue weighs not more than 10 mg (0.2 per cent).
Loss on ignition: Not more than 8.0 per cent, determined on 1.000 g by ignition at 800 °C for 30 min
Calcium Phosphate, Tribasic FCC Food Grade
Tricalcium Phosphate; Precipitated Calcium Phosphate
Calcium Hydroxyapatite
Ca3(PO4)2 Formula weight 310.18
Ca5OH(PO4)3 Formula weight 502.31
Ca10(OH)2(PO4)6 Formula weight 1004.61
INS: 341(iii) CAS 7758-87-4
CAS 1306-06-5
CAS 62974-97-4
DESCRIPTION
Calcium Phosphate, Tribasic, occurs as a white powder that is stable in air. It consists of a variable mixture of calcium phosphates. It is insoluble in alcohol and almost insoluble in water, but it dissolves readily in dilute hydrochloric and nitric acids.
Function: Ant caking agent; buffer; nutrient; clouding agent.
REQUIREMENTS
Identification
A. Add ammonium molybdate TS to a warm solution of sample in a slight excess of nitric acid. A yellow precipitate forms.
B. Dissolve about 100 mg of sample by warming it with 5 mL of 2.7 N hydrochloric acid and 5 mL of water; while shaking, add 1 mL of 6 N ammonium hydroxide, drop-wise and then add 5 mL of ammonium oxalate TS. A white precipitate forms.
Assay: Not less than 34.0% and not more than 40.0% of calcium (Ca).
Arsenic: Not more than 3 mg/kg.
Fluoride: Not more than 0.0075%.
Lead: Not more than 2 mg/kg.
Loss on Ignition: Not more than 10.0%.
The Uses of Tricalcium Phosphate or Calcium Phosphate Tribasic or Calcium Hydroxide Phosphate:
Tricalcium phosphate is a mineral that is used as a supplement in people who do not get enough calcium from food. Calcium phosphate is used to treat calcium deficiencies that may be associated with low blood calcium, a parathyroid disorder, or osteoporosis and other bone conditions. In toothpaste it provides a source of calcium and phosphate ions to support remineralization. It is found in many household items, including baby powder, toothpaste, and antacids.
The MSDS-SDS Hazard Statement of Tricalcium Phosphate or Calcium Phosphate Tribasic or Calcium Hydroxide Phosphate:
Calcium Phosphate Tribasic SDS Safety Data Sheet
Tricalcium Phosphate MSDS Sheet, Material Safety Data Sheet 09-Jan-23
1. Product Identification
Product Name/Synonyms: Calcium Phosphate Tribasic or Tricalcium phosphate; Calcium hydroxy apatite; hydroxyapatite, Precipitated Calcium Phosphate.
CAS No.: 12167-74-7 or 7758-87-4 or 1306-06-5
EINECS EC-No. : 235-330-6 or 231-840-8
Chemical Formula: Ca5(OH)(PO4)3 and also Ca3(PO4)2 ·xH2O
Recommended uses and uses advised against (if any): Industrial Manufacturing.
2. Hazards Identification
GHS, Globally Harmonized System Classification in accordance with 29 CFR 1910
Classification according to Regulation (EC) No 1272/2008
Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008.
This substance is not classified as dangerous according to Directive 67/548/EEC.
Labeling according to GHS USA & Regulation (EC) No 1272/2008
GHS Label Elements None |
Signal Words: None
Precautionary statements:
P261: Avoid breathing dust/fume/gas/mist/vapors/spray.
P262: Do not get in eyes, on skin, or on clothing.
P281: Use personal protective equipment as required.
P302+P352: IF ON SKIN: Wash with plenty of soap and water.
P304 + P340 - IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.
P337+313: If eye irritation persists get medical advice/attention.
3. Composition/Information on Ingredients
Ingredient: Calcium Phosphate Tribasic or Tricalcium Phosphate
CAS No.: 12167-74-7 or 7758-87-4 or 1306-06-5
EINECS EC-No. : 235-330-6 or 231-840-8
4. First Aid Measures
Always seek medical attention after first aid measures are provided.
Inhalation: If inhaled, remove to fresh air. If breathing is difficult, give oxygen. Get medical attention.
Ingestion: Induce vomiting immediately as directed by medical personnel. Never give anything by mouth to an unconscious person.
Skin Contact: Immediately flush skin with plenty of water for at least 15 minutes. Remove contaminated clothing and shoes and wash before reuse. Get medical attention if irritation develops.
Eye Contact: Immediately flush eyes with plenty of water for at least 15 minutes, lifting lower and upper eyelids occasionally. Get medical attention immediately.
5. Fire Fighting Measures
Fire: Not considered to be a fire hazard.
Fire Extinguishing Media: Use any means suitable for extinguishing surrounding fire.
Special Information In the event of a fire, wear full protective clothing and NIOSH-approved self-contained breathing apparatus with full face piece operated in the pressure demand or other positive pressure mode. At high temperatures under fire conditions, it may produce toxic or irritating fumes.
6. Accidental Release Measures
Personal precautions, protective equipment, and emergency procedures: Avoid breathing dust/fumes/gas/mist/vapors/spray. Use individual protective equipment (waterproof boots, suitable protective clothing, safety glasses, etc.). Do not approach facing the wind.
Environmental precautions: Do not let the product enter drains, soil, or water sources.
Methods and materials used for containment Cleanup procedures and Storage: Do not inhale dust, vapors, mist, or gas. Avoid dust formation. Contain spilled material. Cover with an inert, non-combustible absorbent material, (e.g. sand, earth, diatomaceous earth, vermiculite). Use a shovel to put the material into a convenient waste disposal container.
7. Handling and Storage
Precautions for safe handling: Apply according to good manufacturing and industrial hygiene practices. Ensure proper ventilation. In case of insufficient ventilation, wear suitable respiratory equipment. Wash thoroughly after handling. Do not drink, eat, or smoke while handling. Avoid contact with skin, eyes, and clothing. Minimize dust generation. Avoid breathing dust/fumes/gas/mist/vapors/spray. Keep container tightly closed. Avoid ingestion and inhalation. Use individual protective equipment (waterproof boots, suitable protective clothing, safety glasses, etc.).
Conditions for safe storage, including any incompatibilities: Store in cool, dry, and ventilated area away from heat sources and protected from sunlight in tightly closed original container. Keep air contact to a minimum. Store protected from heat, sparks and ignition sources and incompatible materials. Avoid contact with skin and eyes. Avoid inhalation of dust/mist/vapor. Do not store with incompatible materials like strong oxidizing agents.
8. Exposure Controls/Personal Protection
Airborne Exposure Limits: None established.
Ventilation System: A system of local and/or general exhaust is recommended to keep employee exposures as low as possible.
Personal Respirators (NIOSH Approved): For conditions of use where exposure to dust or mist is apparent and engineering controls are not feasible, a particulate respirator may be worn.
Skin Protection: Wear protective gloves and clean body-covering clothing.
Eye Protection: Use chemical safety goggles and/or full face shield where dusting or splashing of solutions is possible. Maintain eye wash fountain and quick-drench facilities in work area.
Other Control Measures: Maintain good housekeeping in work area. Handle in accordance with good industrial hygiene and safety practice.
9. Physical and Chemical Properties
Appearance: Fine, white powder.
Odor: Odorless.
Odor threshold: Not available.
pH: Not available.
Relative density: around 3.14
Melting Point: 1670C (3038F).
Initial boiling point and boiling range: Not available.
Flash point: Not available.
Auto-ignition temperature: Not available.
Decomposition temperature: Not available.
Upper/lower flammability or explosive limits: Not available.
Vapor pressure: Not available.
Vapor density: Not available.
Evaporation rate: Not available.
Flammability (solid, gas): Not available.
Partition coefficient: n-octanol/water: Not available.
10. Stability and Reactivity
Stability: Stable under ordinary conditions of use and storage.
Hazardous Decomposition Products: No information found.
Hazardous Polymerization: Will not occur.
Incompatibilities: Strong oxidizing agents.
Conditions to Avoid: Excessive dust generation.
11. Toxicological Information
Toxicity to Animals: Not available.
Carcinogenicity: No component of this product present at levels greater than or equal to 0.1% is identified as possible or confirmed human carcinogen by IARC, ACGIH, OSHA and NTP.
Mutagenic Effects: Not available.
Developmental Toxicity: Not available.
Reproductive Effects: No information available.
12. Ecological Information
Toxicity to fish: No information available.
Persistence and Degradability: No information available.
Mobility: No information available.
Bioaccumulation/ Accumulation: No information available.
Results of PBT and vPvB assessment: No data available for assessment.
13. Disposal Considerations
Whatever cannot be saved for recovery or recycling should be managed in an appropriate and approved waste disposal facility.
14. Transport Information
DOT (USA): Not controlled.
ADR/RID: Not controlled.
IMDG: Not controlled.
IATA: Not controlled.
15. Regulatory Information
USA:
SARA 311/312: Acute health hazard. See section 2.
DISCLAIMER: The information and recommendations set forth herein are presented in good faith and believed correct as of the date hereof. It is compiled from various sources and it is not necessarily all inclusive nor fully adequate in every circumstance. In addition, these suggestions should not be confused with nor followed in violation of applicable laws, regulations, rules or insurance requirements applicable. This MSDS sheet is intended only as a guide to the appropriate precautionary handling of the material by a properly trained person using this product. Individuals receiving the information must exercise their independent judgment in determining its appropriateness for a particular purpose.
Anmol Chemicals & Pharmaceuticals Pvt. Ltd. is an off-shoot of Anmol Chemicals Taloja. It is located in MIDC Taloja and it is manufacturing pharmaceutical grades of API, Excepients, Food grade and Reagent grade chemicals. Anmol Chemicals & Pharmaceuticals Pvt. Ltd. is a several decades old group of companies, engaged in manufacturing, supplying, distributing, wholesale supplies for actual users, retail or small pack supplies for research and development chemicals, fine and speciality chemicals, pharmaceutical excipients, mineral fortifiers in chemically pure, Analytical reagent grade, IP BP USP Ph Eur EP JP and other pharmaceutical grade monograph including FCC Food grade chemicals and Nutraceuticals, Mineral Fortifiers at best prices.
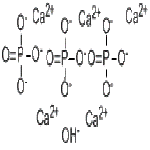
Tricalcium Phosphate or Calcium Phosphate Tribasic or Calcium Hydroxide Phosphate Structure
CAS Number 7758-87-4 or 12167-74-7 or 1306-06-5 or 62974-97-4, Tricalcium Phosphate or Calcium Phosphate Tribasic or Calcium Hydroxide Phosphate Manufacturer Exporter
ANMOL CHEMICALS & PHARMACEUTICALS Pvt. Ltd.
India, USA, Europe, UAE
TELEPHONE: +912223770100
Navi Mumbai, INDIA
e-mail: info(At the rate i.e. @)anmol.org
Copyright. 22-nov-24
We manufacture:
Glacial Acetic Acid Manufacturer
Calcium Gluceptate or Calcium Glucoheptonate
Calcium Magnesium Lactate Gluconate

